Discover relevant documents in Dea drug Order Forms online catalog and effortlessly edit them online. Have complete control of your documents and ensure document safety and compliance without difficulty.

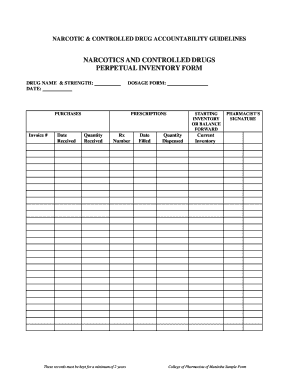















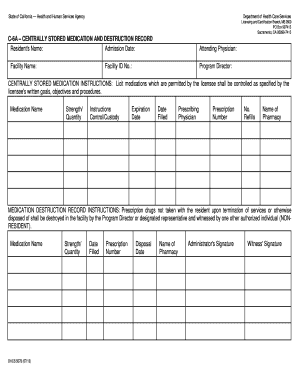








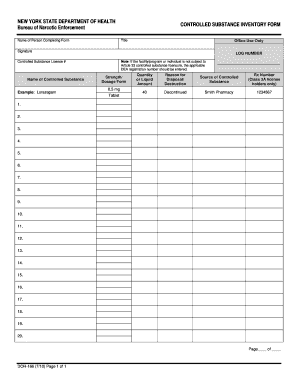

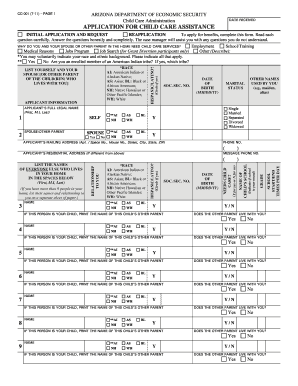








Papers management takes up to half of your business hours. With DocHub, it is possible to reclaim your office time and enhance your team's productivity. Access Dea drug Order Forms collection and check out all templates relevant to your daily workflows.
Easily use Dea drug Order Forms:
Accelerate your daily document management using our Dea drug Order Forms. Get your free DocHub account today to explore all templates.