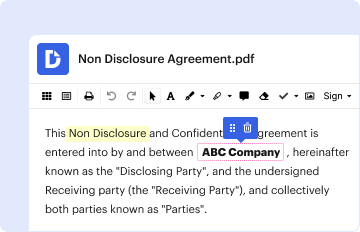
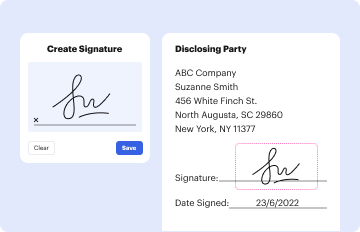
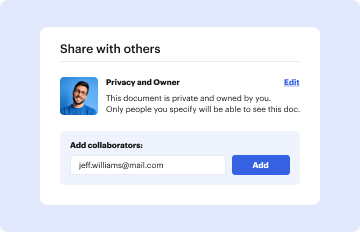
Definition and Meaning
The "Schedules and Recommendation" document is designed to provide a structured and detailed framework for understanding scheduling and recommendations related to various processes. It often serves as an authoritative guide for best practices in scheduling tasks, appointments, or events and recommending actions based on specific criteria or assessments. This type of document is commonly used in fields that require precise timing and procedural guidance, such as healthcare, project management, and educational planning. By delivering comprehensive insights into what needs to be scheduled and recommended, the document helps users streamline operations and improve efficiency.
How to Use the Schedules and Recommendation
Utilizing the "Schedules and Recommendation" document involves several straightforward steps:
-
Review the Document: Begin by thoroughly reading the document to understand the scope and objectives. It is essential to grasp the entire context to maximize utility.
-
Identify Relevant Sections: Locate the sections pertinent to your needs. For example, if the document involves vaccination schedules, focus on areas relevant to your requirements, such as adult health or pediatric care.
-
Apply Recommendations: Implement the recommendations offered in the document by integrating them into your workflow. This may involve making timing adjustments, prioritizing tasks, or consulting additional resources specified within the document.
-
Monitor Outcomes: Continuously assess the results of your applied strategies to ensure they yield desired outcomes. Revise approaches if necessary to align with updated guidance or unforeseen variables.
How to Obtain the Schedules and Recommendation
The "Schedules and Recommendation" document can be acquired through various channels:
- Official Websites: Frequently, institutions or organizations that produce such documents offer them on their websites for download.
- Professional Associations: Members of field-specific associations may distribute these documents via their networks.
- Conferences and Seminars: Attendees can often access special editions or updates of such documents when participating in industry events.
- Direct Requests: Contacting the issuing body directly to request copies or clarification can also be an effective way to obtain the latest version.
Steps to Complete the Schedules and Recommendation
Completing tasks outlined in the "Schedules and Recommendation" document usually involves:
-
Data Collection: Gather necessary information, such as relevant dates, timelines, or specific criteria, pertinent to your scheduling or recommendation needs.
-
Draft Creation: Begin drafting your schedule or recommendation based on collected data, utilizing templates if provided by the document.
-
Review and Edit: Cross-check the draft against the guidelines and specifications given in the document. Make necessary adjustments to comply with detailed instructions.
-
Finalization: Conclude the process by formalizing the completed schedule or recommendation. Ensure that all sections are filled out accurately and that the document is ready for distribution or implementation.
Key Elements of the Schedules and Recommendation
Several critical elements are often included in the "Schedules and Recommendation" document:
- Objective and Scope: Defines the purpose and coverage area of the document.
- Scheduling Details: Includes timelines, deadlines, and sequencing for tasks or events.
- Recommendation Criteria: Lists standards or benchmarks that guide decision-making.
- Procedural Steps: Outlines specific steps to enforce guidelines effectively.
- Feedback Mechanisms: Provides ways to measure outcomes and incorporate feedback for continuous improvement.
Legal Use of the Schedules and Recommendation
The legal application of the "Schedules and Recommendation" document typically involves ensuring compliance with the regulations and standards established by governing bodies. In some industries, adherence to these documents is mandatory to avoid penalties or legal disputes. For instance, healthcare schedules related to immunization are backed by public health laws, while staff schedules in regulated professions must comply with labor laws. Understanding the legal context and implications of the document helps align your processes with compliance requirements and uphold good practices.
Examples of Using the Schedules and Recommendation
Real-world scenarios of utilizing the "Schedules and Recommendation" document:
- Healthcare Settings: Medical facilities use vaccine schedules to plan patient immunizations, ensuring public health safety and meeting legal compliance.
- Project Management: Project managers rely on scheduling recommendations to allocate resources efficiently and meet critical deadlines.
- Education Systems: School administrations implement recommended schedules to organize the academic year, optimizing learning outcomes and resource utilization.
Software Compatibility
The "Schedules and Recommendation" document, depending on its format, can be compatible with various software applications. This ensures users can leverage technology to streamline interactions with the document:
- PDF Editors like Adobe Acrobat: Useful for viewing and annotating the document.
- Spreadsheet Applications like Excel: Can organize schedules or recommendations into sortable data formats.
- Project Management Tools like Microsoft Project: For integrating scheduling elements into broader project timelines.
- Document Sharing Platforms like Google Drive: Allow for collaborative updates and access to the latest versions among teams.
Compatibility with such platforms enhances the efficiency of using the document and enables easier collaboration and data management.