
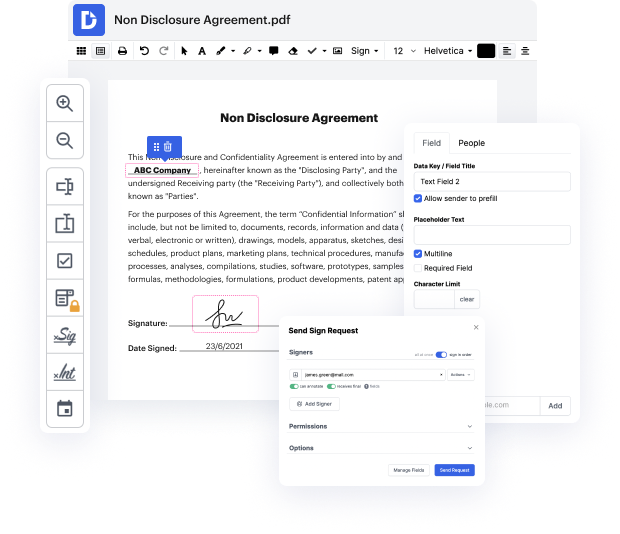
Are you having a hard time choosing a trustworthy option to Open Label Notification For Free? DocHub is set up to make this or any other process built around documents much easier. It's straightforward to navigate, use, and make edits to the document whenever you need it. You can access the core features for dealing with document-based workflows, like certifying, importing text, etc., even with a free plan. In addition, DocHub integrates with different Google Workspace apps as well as services, making document exporting and importing a piece of cake.
DocHub makes it easier to edit paperwork from wherever you’re. Plus, you no longer need to have to print and scan documents back and forth in order to sign them or send them for signature. All the essential features are at your fingertips! Save time and hassle by executing paperwork in just a few clicks. Don’t wait another minute today!
The speaker expresses gratitude for the invitation to present at a medical conference on anxiety disorders. They share preliminary data from a CBD clinical trial for anxiety. Anxiety disorders are common globally, with one in 13 people affected. Prevalence varies by age group, with those aged 30-44 having the highest likelihood of anxiety disorders in the US. The speaker mentions the impact of the COVID-19 pandemic on anxiety levels.