
Are you searching for a simple way to embed tag in Clinical Trial Agreement Template? DocHub provides the best solution for streamlining form editing, signing and distribution and form execution. With this all-in-one online program, you don't need to download and set up third-party software or use complex file conversions. Simply upload your form to DocHub and start editing it quickly.
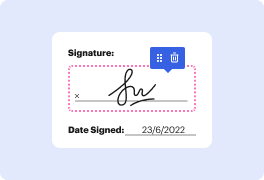
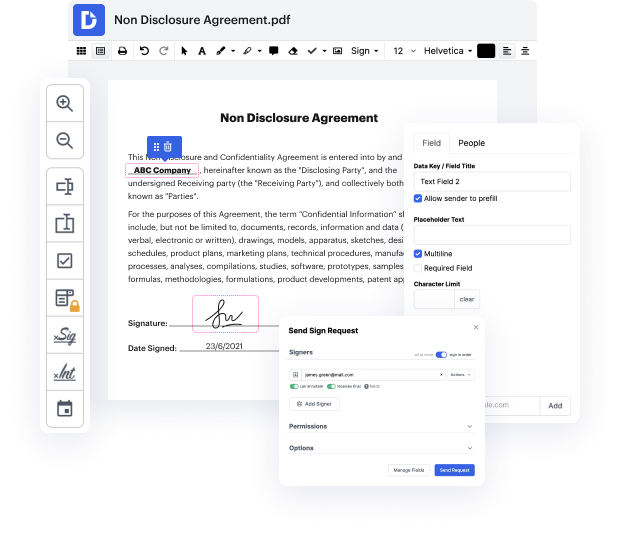
DocHub's drag and drop user interface allows you to swiftly and easily make modifications, from intuitive edits like adding text, photos, or graphics to rewriting whole form pieces. In addition, you can endorse, annotate, and redact paperwork in just a few steps. The solution also allows you to store your Clinical Trial Agreement Template for later use or convert it into an editable template.
DocHub offers beyond you’d expect from a PDF editing system. It’s an all-encompassing program for digital form management. You can utilize it for all your paperwork and keep them safe and easily accessible within the cloud.
hows it going guys my name is dom and today im going to be showing you quite possibly the easiest way to embed pdfs into your websites now this solution right here is going to require no external libraries or frameworks its all going to be done natively and it really is extremely simple all right so right here i have this new html document with nothing inside the body now i assume you guys might be coming from an existing project or website and you are looking to embed the pdf into that website if you are dont worry it should work perfectly fine all right now were going to be embedding this sample.pdf the same one that i showed you at the beginning of todays video so very simple pdf document right here and a quick shout out to this vs code extension called pdf viewer by mathematic i found this right here just a really simple and easy to use extension and it basically worked right away so without this i would be showing you guys the pdf source code and not the actual visual previe