Definition and Significance of the Investigational Medicinal Product Dossier
An Investigational Medicinal Product Dossier (IMPD) is a pivotal document in the field of pharmaceutical development. It encompasses all essential data regarding a medicinal product intended for use in clinical trials. The IMPD is designed to present comprehensive information concerning the quality, safety, and efficacy of the investigational product. It serves as a crucial reference for regulatory authorities, outlining the complexities of the drug’s formulation, manufacturing process, and preclinical results.
Key Components of an IMPD
-
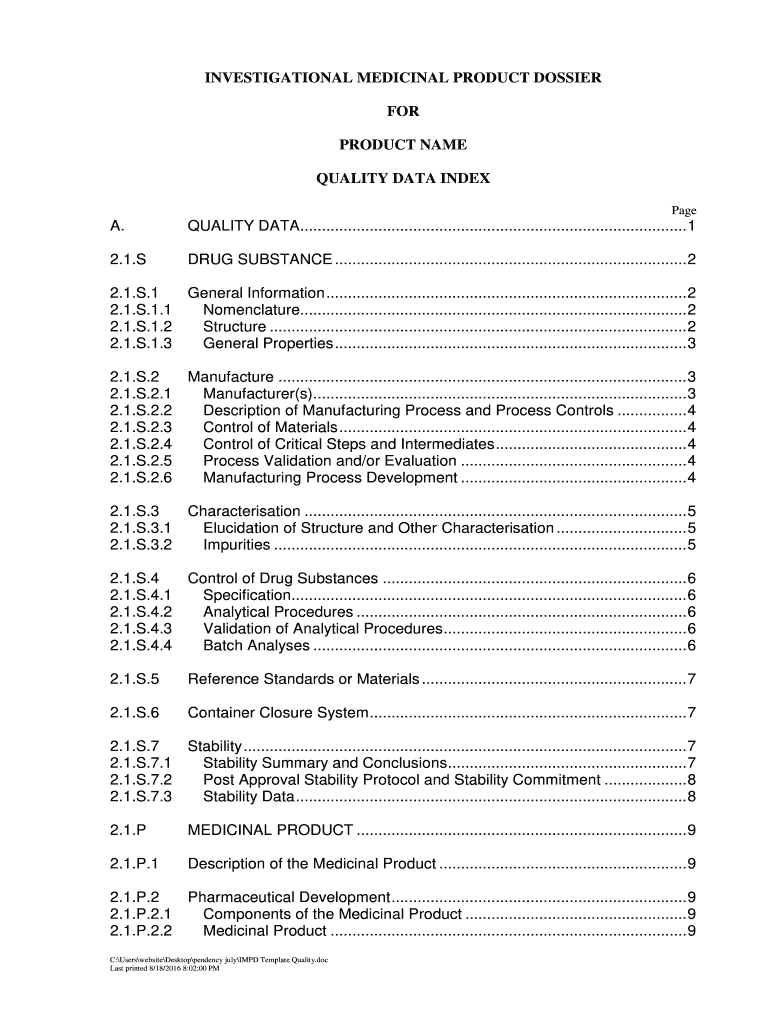
Quality Data: Detailed sections on the drug substance and product, including information on the manufacturing processes, characterization, and quality control measures.
-
Clinical and Non-Clinical Data: Comprehensive reports on preclinical studies and clinical trial protocols, elucidating how the product has been tested for safety and efficacy.
-
Regulatory Compliance: The dossier must comply with the guidelines set by regulatory bodies, such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe.
This detailed structure ensures that regulatory authorities can fully evaluate the investigational product's safety and efficacy before approving it for human trials.
Steps to Prepare an Investigational Medicinal Product Dossier
Creating an effective IMPD requires a structured approach. The following steps provide a foundation for preparing a thorough dossier:
-
Gather Necessary Documentation: Compile all related documents, including product formulation, manufacturing methods, and previous research data.
-
Conduct Stability Testing: Include extensive stability data that demonstrates the product’s shelf life and efficacy over time.
-
Ensure Quality Control Measures: Document all quality control processes, including batch testing outcomes, to illustrate compliance with regulatory standards.
-
Compile Clinical and Safety Reports: Include summaries of preclinical and clinical trials, detailing the safety profiles and pharmacology of the investigational product.
-
Review Regulatory Guidelines: Familiarize yourself with the relevant guidelines provided by regulatory authorities to ensure compliance and completeness.
-
Draft the IMPD: Organize the information into the designated sections of the dossier, ensuring clarity and conciseness.
-
Internal Review: Conduct a thorough internal review of the completeness and accuracy of the dossier before submission.
-
Submit the Dossier: Depending on the jurisdiction, submit the IMPD electronically or in printed format to the relevant regulatory body.
Following these steps meticulously will enhance the likelihood of an efficient review process by regulatory agencies.
Common Users of the Investigational Medicinal Product Dossier
The IMPD is utilized extensively across various sectors within the pharmaceutical and biotechnology industries. Common users include:
-
Pharmaceutical Companies: Organizations involved in the development and manufacturing of investigational medicinal products utilize the dossier to meet regulatory compliance.
-
Biotechnology Firms: Similar to pharmaceutical companies, these firms require an IMPD to detail the biotechnology-derived products under investigation.
-
Research Institutions: Universities and research organizations conducting clinical trials must prepare an IMPD to present their findings and methodologies.
-
Regulatory Authorities: Agencies like the FDA and EMA review IMPDs as part of the clinical trial approval process, making them essential for those bodies.
These users rely on the IMPD to guide the development of safe and effective medicinal products while ensuring transparency with regulatory agencies.

Important Terms Related to the Investigational Medicinal Product Dossier
Understanding key terminologies associated with the IMPD is vital for stakeholders involved in pharmaceutical development:
-
Investigational Product: A medicinal product that is being tested in a clinical trial and has not yet received final regulatory approval.
-
Non-Clinical Studies: Research conducted on non-human subjects to gather safety and efficacy data before clinical trials.
-
Clinical Trials: Research conducted to assess the safety and efficacy of a medicinal product in human subjects.
-
Regulatory Submission: The process of submitting the IMPD and other necessary documents to regulatory authorities for review and approval.
Familiarizing oneself with these terms is crucial for facilitating effective communication among professionals engaged in the development and regulation of medicinal products.
Key Elements of an Investigational Medicinal Product Dossier
A well-structured IMPD comprises several critical elements that must be addressed thoroughly:
-
Introduction and General Information: This section should outline the purpose of the IMPD, details of the investigational product, and its intended use.
-
Quality Information: Provide detailed descriptions of the quality of the investigational medicinal product, including raw materials, formulation, and manufacturing processes.
-
Preclinical and Clinical Data: Summarize all non-clinical studies, including pharmacodynamics, pharmacokinetics, and safety evaluations, followed by clinical trial information.
-
Regulatory Status: Include information on any previous submissions or approvals from regulatory authorities relating to the investigational product.
By encompassing these elements, the IMPD will serve as a comprehensive document that facilitates informed decision-making by regulatory authorities and stakeholders alike.
Examples of Using the Investigational Medicinal Product Dossier
The IMPD has practical applications across various stages of drug development. Consider the following scenarios:
-
New Drug Application (NDA): A pharmaceutical company seeking to launch a new drug must prepare an IMPD that outlines the quality, safety, and efficacy data necessary for the NDA submission to the FDA.
-
Clinical Trial Approval: A biotech firm may submit an IMPD to obtain approval for a Phase I clinical trial, providing in-depth descriptions of their investigational product’s formulation, manufacturing processes, and initial safety data.
-
Regulatory Inquiries: In cases where regulatory authorities require more information about a product, an IMPD can serve as a source for clarification on any aspects of the medicinal product’s quality and efficacy.
These examples highlight the critical role of the IMPD in navigating the complex landscape of drug development and regulatory compliance.