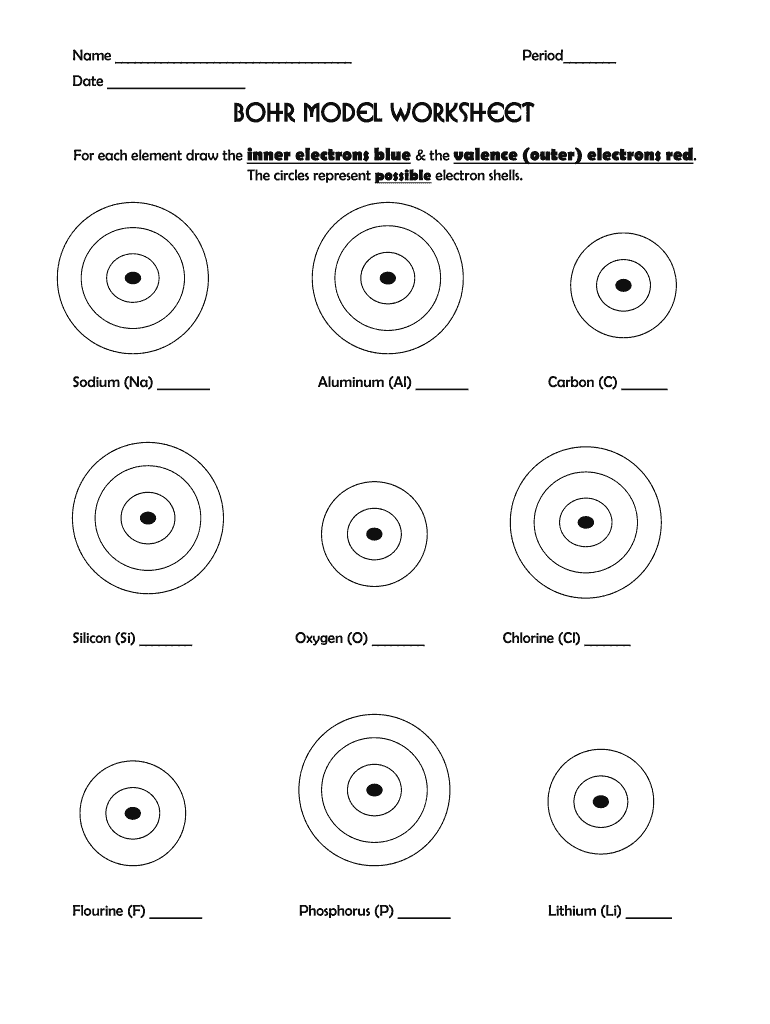
Definition and Meaning of the Bohr Model Worksheet
The Bohr model worksheet is an educational tool designed to help students understand the Bohr model of atomic structure. This model describes the arrangement of electrons around the nucleus of an atom. The worksheet typically includes various exercises that require students to illustrate electron configurations for different elements, calculate the number of protons, neutrons, and electrons, and complete tables detailing the properties of sub-atomic particles. Such worksheets are essential for reinforcing concepts of atomic theory and aiding students in visualizing electron orbits.
Components of the Bohr Model Worksheet
- Electron Configurations: Students learn to determine how electrons are distributed in different energy levels.
- Subatomic Particles: Worksheets often include tables where students must list and calculate the number of protons, neutrons, and electrons for given elements.
- Bohr Diagrams: Students are tasked with drawing Bohr diagrams that represent the atomic structure visually, aiding in comprehension of electron arrangements.
How to Use the Bohr Model Worksheet Effectively
To get the most out of a Bohr model worksheet, following a clear strategy can enhance the learning experience.
- Review the Bohr Model: Familiarize yourself with the fundamental principles of the Bohr model, including energy levels and electron behavior.
- Step-by-Step Approach: Tackle each section of the worksheet methodically — start with defining terms, then move on to drawing diagrams.
- Utilize Resources: Refer to textbooks or online resources to clarify concepts related to atomic structure and electron configuration.
- Practice Regularly: Consistent practice with different elements will reinforce learning and aid in retention of the material.
Steps to Complete the Bohr Model Worksheet
Completing a Bohr model worksheet can be broken down into a sequence of manageable steps:
- Identify the Element: Determine which element you are working with, often specified in the worksheet. Look up its atomic number and mass.
- Calculate Subatomic Particles: Use the atomic number to identify the number of protons. Subtract the atomic number from the atomic mass to find the number of neutrons. The number of electrons will equal the number of protons for neutral atoms.
- Draw the Bohr Diagram: Start by sketching a nucleus with protons and neutrons. Then, draw circles around the nucleus representing electron orbits, filling them according to the number of electrons and their energy levels.
- Answer Questions: Respond to any questions related to the properties of the element based on your calculations and diagram.
Important Terms Related to the Bohr Model Worksheet
Understanding specific terminology is crucial when working with the Bohr model worksheet. Familiarity with these terms will aid in effectively utilizing the worksheet.
- Atomic Number: This represents the number of protons in the nucleus of an atom, which also indicates the number of electrons in a neutral atom.
- Atomic Mass: The total mass of an atom, reflecting the sum of protons and neutrons.
- Electron Configuration: The distribution of electrons in an atom's energy levels and orbitals.
- Bohr Diagram: A visual representation of an atom, illustrating the nucleus and the arrangement of electrons.
Examples of Using the Bohr Model Worksheet in Education
Bohr model worksheets can be integrated into various educational practices to reinforce learning.
- Classroom Activities: Teachers can use these worksheets during labs or discussions on atomic theory. Group activities can encourage collaboration among students.
- Homework Assignments: Assigning worksheets as homework allows students to practice independently, solidifying their understanding of atomic structures.
- Test Preparation: Students can utilize practice worksheets to prepare for quizzes or exams that cover atomic theory and electron configurations.
- Supplemental Learning: Providing these worksheets as additional resources can assist students who may need extra help grasping complex concepts in chemistry.
Understanding the Bohr model through effective use of worksheets promotes engagement and comprehension, facilitating a solid foundation in atomic theory.