Overview of Clarity® Urine/HCG Liquid Controls Quality Control Log
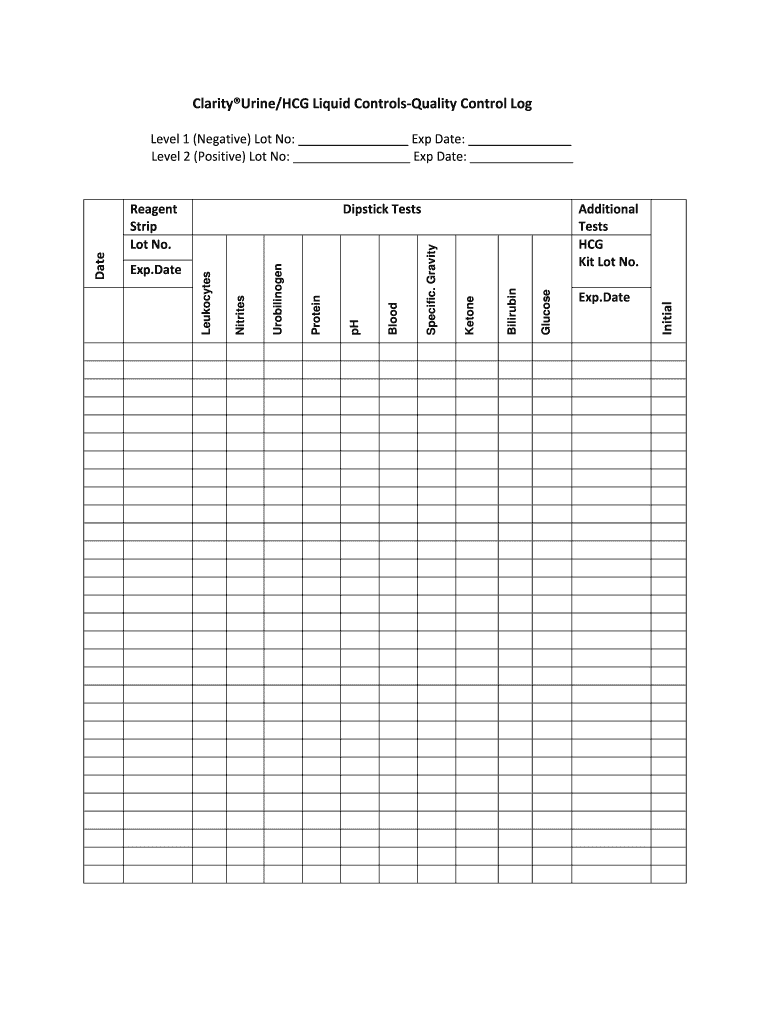
The Clarity® Urine/HCG Liquid Controls Quality Control Log is essential for maintaining accuracy and reliability in urinalysis testing. This log serves as a detailed record for laboratory personnel, facilitating consistent monitoring of test performance through systematic documentation of results.
Key Parameters to Record in the Log
Detailed parameters that are crucial for quality control in urine testing include:
- Urobilinogen: The measurement of urobilinogen is important for diagnosing liver function and hemolytic disorders.
- Protein: Elevated protein levels can indicate kidney damage or disease.
- pH: Analyzing urine pH assists in identifying metabolic or respiratory issues.
- Blood: The presence of blood may indicate urinary tract infections or kidney stones.
- Specific Gravity: This parameter reflects hydration status and kidney concentrating ability.
- Ketone: Detection of ketones is vital in evaluating diabetes management.
- Bilirubin: Bilirubin levels can indicate liver dysfunction or bile duct obstruction.
- Glucose: Presence of glucose in urine often suggests uncontrolled diabetes.
- Nitrites: Testing for nitrites helps in confirming bacterial infections in the urinary tract.
- Leukocytes: The presence of leukocytes indicates potential infection or inflammation.
Documentation Features of the Quality Control Log
The design of the Clarity® Urine/HCG Liquid Controls Quality Control Log incorporates several essential features to ensure thorough documentation:
- Reagent Strip Lot Numbers: This section allows users to record the lot numbers of reagent strips used, which is critical for traceability and accountability.
- Expiration Dates: Recording expiration dates ensures that only valid reagents are used in testing, maintaining quality standards.
- Initial Testing Levels: This section captures baseline data for both negative and positive controls, providing a reference point for future tests.
Importance of Accurate Record-Keeping
Accurate record-keeping is critical in laboratory settings for various reasons:
- Regulatory Compliance: Adequate documentation aligns with laboratory standards and regulations, ensuring compliance during audits.
- Test Reliability: Maintaining a quality control log supports the reliability and validity of test results, allowing for strategic improvements in testing processes.
- Error Tracking: By documenting results, laboratories can identify trends or recurring issues, facilitating corrective actions efficiently.
Common Practices for Using the Quality Control Log
To effectively utilize the quality control log, laboratories should adopt the following practices:
- Regular Updates: Ensure the log is updated with each test conducted, promoting accuracy and accountability.
- Centralized Access: Store the log in a central location accessible to all laboratory personnel, ensuring transparency and collaborative checking of data.
- Training: Provide newcomers with comprehensive training on the importance of quality control logs and how to fill them out correctly. This aids in maintaining consistency and reducing errors.
Examples of Quality Control Scenarios
Several scenarios may arise that underscore the importance of the Clarity® Urine/HCG Liquid Controls Quality Control Log, including:
- Unexpected Results: If an initial test returns inconclusive results, reviewing the quality control log can elucidate whether reagents or procedural errors contributed to the outcome.
- Quality Assurance Audits: An audit may necessitate reviewing historical quality control logs to verify compliance with established testing protocols.
- Trend Analysis: By examining logged data over time, laboratories can identify patterns that may warrant operational adjustments for improved testing efficiency.
Best Practices for Maintaining Urinalysis Quality Control Logs
To ensure the integrity and usefulness of the log, consider the following best practices:
- Consistent Review: Regularly review the log for completeness and accuracy, ensuring any discrepancies are addressed immediately.
- Backup Procedures: Implement methods for securely backing up digital logs or preserving paper copies, safeguarding against data loss.
- Integration with Other Logs: If applicable, integrate the urinalysis quality control log with other laboratory logs for a holistic view of lab operations and test performance.
Maintaining a robust quality control log for Clarity® Urine/HCG Liquid Controls is a foundational element in ensuring accurate and reliable urinalysis testing procedures. By documenting vital parameters and adhering to best practices, laboratories can enhance their testing integrity and compliance.