

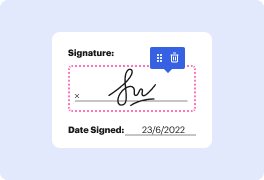

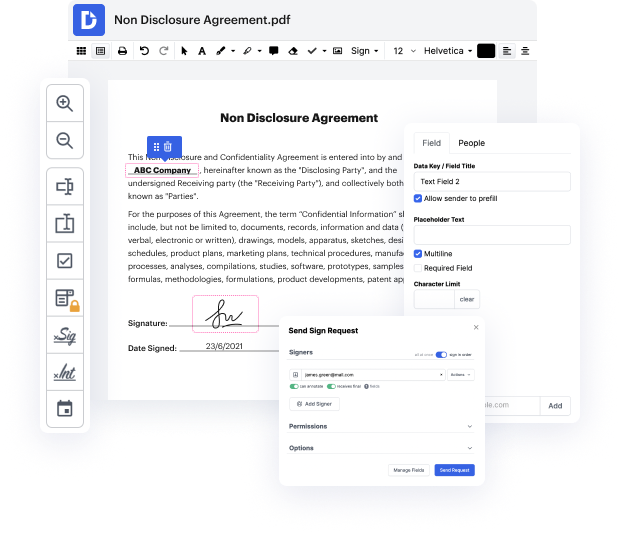
You no longer have to worry about how to work in shape in dot. Our powerful solution guarantees easy and fast document management, enabling you to work on dot documents in a few minutes instead of hours or days. Our platform contains all the features you need: merging, adding fillable fields, approving forms legally, adding symbols, and so on. There’s no need to set up additional software or bother with high-priced applications requiring a powerful device. With only two clicks in your browser, you can access everything you need.
Start now and handle all various types of forms professionally!
professor Dave here, letamp;#39;s learn how to draw Lewis dot structures weamp;#39;ve learned about how ions come together to form ionic compounds but much more interesting is the way atoms form networks of covalent bonds. in order to talk about this weamp;#39;re going to have to learn how to draw Lewis dot structures when we draw these we will represent an atom by its chemical symbol. hereamp;#39;s carbon. we then draw that atomamp;#39;s valence electrons as dots around the atom. carbon is one 1s2 2s2 2p2 so thatamp;#39;s four electrons in the outermost shell, which you can also tell by counting over from the left for carbon and other small atoms we assume that there are four coordination sites that can accommodate electrons or bonds because this is how carbon can fill its octet, or its outermost shell. when we draw the valence electrons we put one in each coordination site first before pairing them up so carbon will look like this not like this elements in the same group will h
At DocHub, your data security is our priority. We follow HIPAA, SOC2, GDPR, and other standards, so you can work on your documents with confidence.
Learn more



