
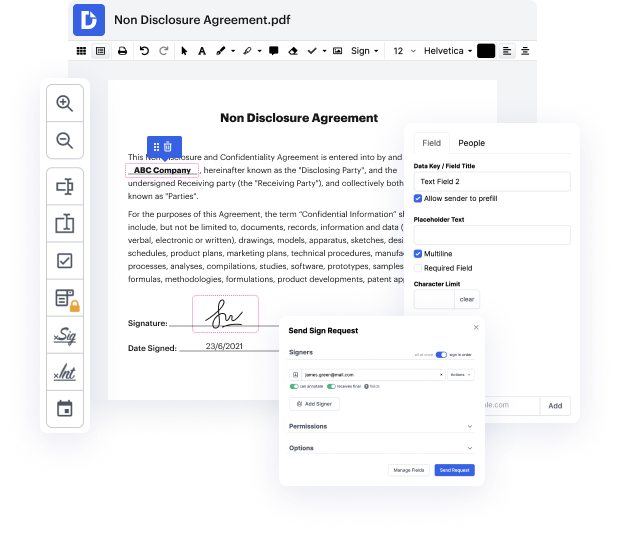
DocHub is an all-in-one PDF editor that enables you to work in issue in INFO, and much more. You can underline, blackout, or erase paperwork elements, insert text and images where you need them, and collect information and signatures. And since it works on any web browser, you won’t need to update your hardware to access its robust tools, saving you money. With DocHub, a web browser is all you need to make changes in your INFO.
Sign in to our website and follow these instructions:
It couldn't be easier! Streamline your document management now with DocHub!
in this video weamp;#39;re going to focus on chemistry problems related to internal energy heat and work so letamp;#39;s start with this one calculate the change in the internal energy of a system if 300 joules of heat energy is absorbed by the system and if 400 joules of work is done on the system now i donamp;#39;t know if you saw a previous video that i created on the first law of thermodynamics but if you havenamp;#39;t in chemistry delta u the change in internal energy is equal to q plus w in physics itamp;#39;s q minus w now q is positive whenever heat is absorbed by the system so thatamp;#39;s during an endothermic process q is negative whenever heat is released by the system so thatamp;#39;s during an exothermic process w is positive whenever work is done on a system so the internal energy of the system will go up and w is negative whenever work is done by the system so this would decrease the internal energy of the system so those are a few things to keep in mind throug