
When you deal with diverse document types like Clinical Trial Agreement Template, you know how significant accuracy and attention to detail are. This document type has its specific format, so it is crucial to save it with the formatting intact. For this reason, working with this kind of paperwork can be quite a challenge for conventional text editing software: a single incorrect action might mess up the format and take additional time to bring it back to normal.
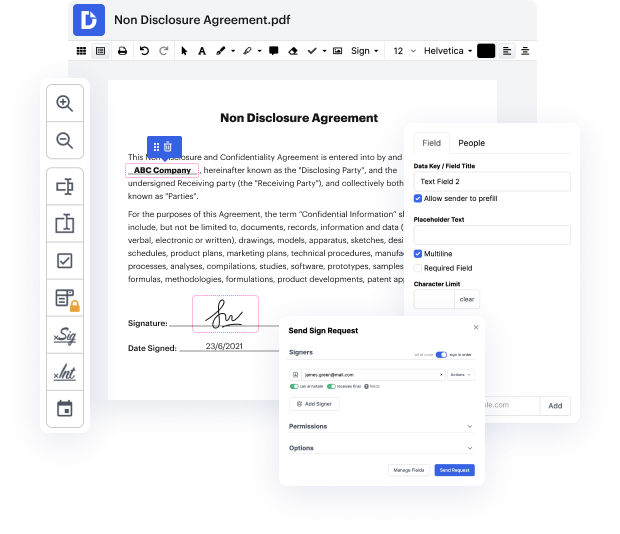
If you wish to tack phone in Clinical Trial Agreement Template without any confusion, DocHub is a perfect instrument for such duties. Our online editing platform simplifies the process for any action you may want to do with Clinical Trial Agreement Template. The streamlined interface design is suitable for any user, whether that individual is used to working with such software or has only opened it for the first time. Access all modifying tools you require easily and save your time on everyday editing activities. All you need is a DocHub profile.
See how easy document editing can be irrespective of the document type on your hands. Access all top-notch modifying features and enjoy streamlining your work on documents. Sign up your free account now and see immediate improvements in your editing experience.
In this video, the speaker discusses the impact of CTA negotiations on the clinical trials industry, highlighting the importance of quick access to medicines for patients. They suggest that CTA delays are not solely due to one issue but involve various components, which they refer to as "villains." The speaker shares that they have written a book on this topic, which will be released soon in installments. The aim is to address the challenges and complexities of CTA negotiations in the industry.