
Are you looking for a straightforward way to strike point in Clinical Trial Agreement Template? DocHub offers the best platform for streamlining form editing, signing and distribution and document endorsement. With this all-in-one online platform, you don't need to download and install third-party software or use complex document conversions. Simply upload your form to DocHub and start editing it quickly.
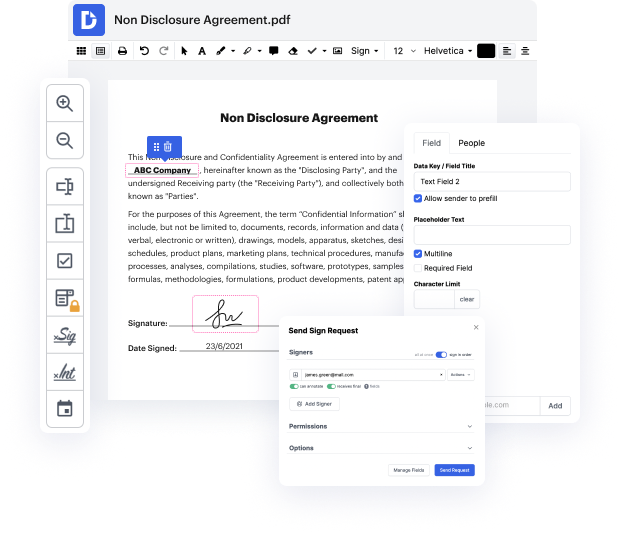
DocHub's drag and drop user interface enables you to swiftly and quickly make changes, from simple edits like adding text, images, or graphics to rewriting entire form components. In addition, you can sign, annotate, and redact papers in just a few steps. The editor also enables you to store your Clinical Trial Agreement Template for later use or convert it into an editable template.
DocHub offers more than just a PDF editing system. It’s an all-encompassing platform for digital form management. You can use it for all your papers and keep them secure and swiftly readily available within the cloud.
The fall clinical trials toolkit series is launched with a focus on clinical trial agreement review and negotiation. Today's session features Rachel Humberson, the interim assistant director from the research office, and Julie, a negotiator with the office of sponsored programs. Participants are reminded that continuing education credits are available for WVU employees; interested individuals should provide their names and email addresses in the chat for attendance. Audience members are encouraged to submit questions in the chat, which will be relayed to the speakers during the session.