
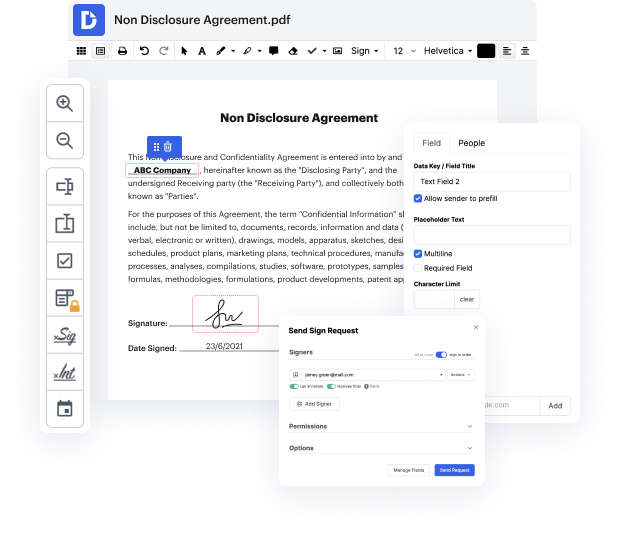
Having full power over your papers at any moment is essential to relieve your day-to-day duties and improve your efficiency. Accomplish any objective with DocHub features for papers management and hassle-free PDF editing. Gain access, adjust and save and incorporate your workflows along with other protected cloud storage services.
DocHub provides you with lossless editing, the chance to use any format, and securely eSign documents without looking for a third-party eSignature software. Make the most from the document managing solutions in one place. Consider all DocHub features right now with the free of charge account.
Before participating in a study, it's essential to understand its details and what participation involves. Researchers will provide an informed consent document, which includes information about the study's duration, required visits, medications, and medical procedures. It outlines expected outcomes, potential benefits, risks, and other relevant details. A translator may be available if needed. Researchers will review the document with you and answer any questions. After you have all the necessary information and discussions with staff and family, you can decide whether to participate. If you agree, you'll sign the informed consent statement, affirming your understanding and voluntary participation, with the option to withdraw at any time.