
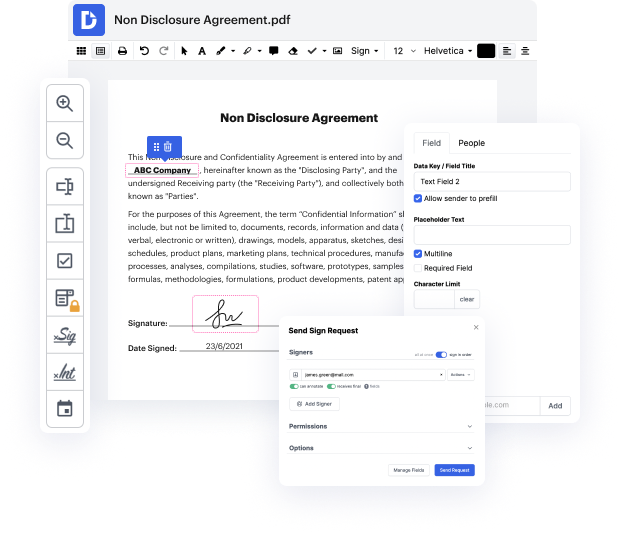
Are you looking for an easy way to slide attachment in NDA? DocHub provides the best platform for streamlining document editing, certifying and distribution and form execution. With this all-in-one online platform, you don't need to download and set up third-party software or use complex file conversions. Simply upload your document to DocHub and start editing it in no time.
DocHub's drag and drop user interface allows you to swiftly and quickly make tweaks, from simple edits like adding text, pictures, or graphics to rewriting entire document pieces. In addition, you can sign, annotate, and redact documents in a few steps. The editor also allows you to store your NDA for later use or transform it into an editable template.
DocHub provides beyond you’d expect from a PDF editing program. It’s an all-encompassing platform for digital document management. You can use it for all your documents and keep them safe and swiftly readily available within the cloud.
everyone my name is elizabeth jaquinto friedman and im a regulatory counsel in the division of policy development in the office of generic drug policy today im going to provide an overview on evaluating therapeutic equivalence there are three main learning objectives for this presentation and those objectives are to outline the fundamentals of therapeutic equivalence describe what fda considers in therapeutic equivalence evaluations and describe how fda makes therapeutic equivalence determinations well move right into the fundamentals of therapeutic equivalence drug products that are therapeutic equivalents can be substituted with the full expectation that the substituted product will produce the same clinical effect and safety profile as the prescribed product therapeutic equivalence evaluations are listed in the fda publication approved drug products with therapeutic equivalence evaluations better known as the orange book for multi-source prescription drug products approved under