
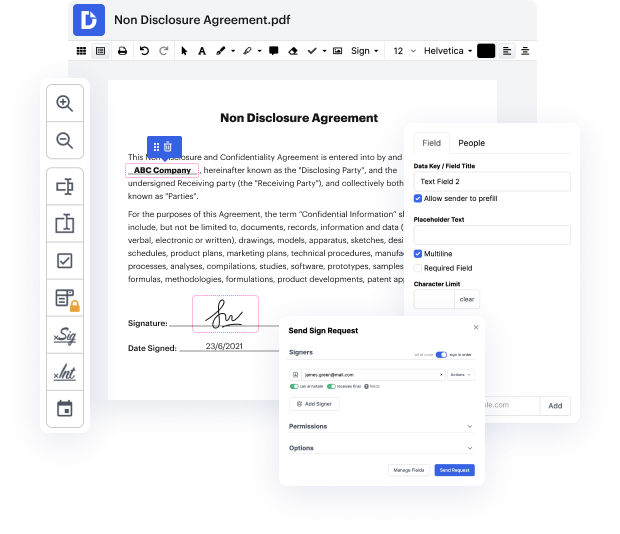
Managing and executing papers can be tedious, but it doesn’t have to be. No matter if you need help day-to-day or only occasionally, DocHub is here to supply your document-based tasks with an extra productivity boost. Edit, comment, complete, sign, and collaborate on your Clinical Trial Agreement Template quickly and effortlessly. You can adjust text and images, build forms from scratch or pre-built web templates, and add eSignatures. Due to our top-notch security measures, all your data remains secure and encrypted.
DocHub provides a complete set of capabilities to simplify your paper processes. You can use our solution on multiple systems to access your documents anywhere and whenever. Improve your editing experience and save hours of handiwork with DocHub. Try it for free right now!
guru nation thank you so much for watching make sure you like subscribe comment share it means a lot to me keep sending your questions your comments uh anything helps but some of them also help for creating the content like todays video and i cant believe i never had a video i probably have in the past but recently just on its own about study startup and study startup this is going to be helpful whether youre a site or whether youre somebody trying to learn the ins and outs of being a cra when it comes to study startup so im actually going through this right now with my site we have a new study im the coordinator so im going to share from my perspective as a coordinator and also from my experiences in the past as a cra as well with study startup so once the site has been awarded a study the cra sends the or the sponsor or somebody sponsor cro or cra send the site acceptance letter uh or theyre just sent the startup packet and the acceptance letter is in an email saying hey you