
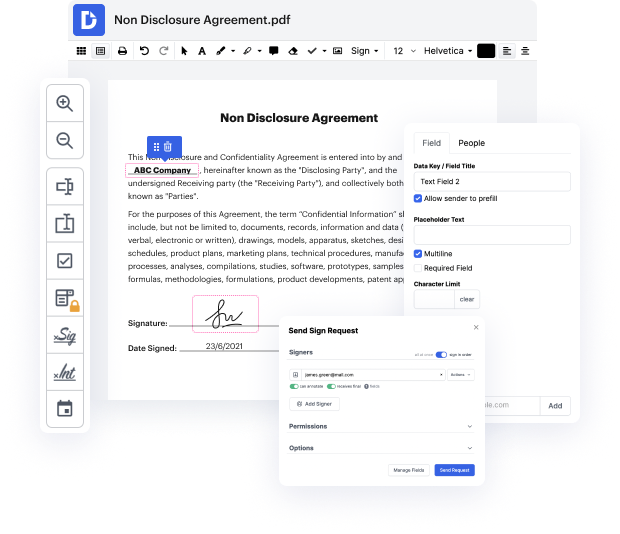
Manual document processing can be quite a reason for your enterprise losing funds and your staff members losing interest in their duties. The simplest way to accelerate all organization processes and increase your data is to manage everything with cutting-edge software like DocHub. Deal with all your documents and Save Change In Control Agreement in DOC within just seconds and save more time for pertinent duties.
With DocHub, you have unrestricted access to your documents and Templates available for you at any time. Explore all capabilities today with your free of charge DocHub account.
Rob Packard from Medical Device Academy discusses the distinctions between document change control and design change control processes. He highlights a common mistake among clients who, while learning document change control for their new quality systems, overlook the specific needs for design change control, particularly since many are introducing medical devices for the first time. Packard emphasizes that while it may seem logical to apply document control processes to approve various changes, the nuances of design changes—such as modifications to devices, manufacturing processes, or suppliers—require a distinct approach. Proper understanding and application of each process are crucial for successful market entry.