
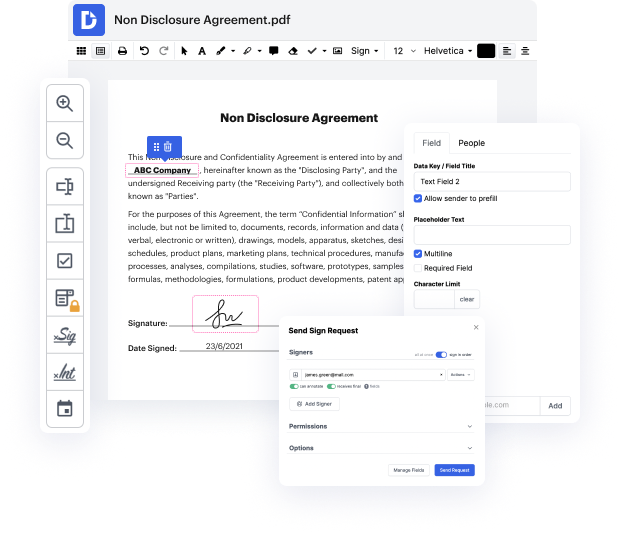
Disadvantages are present in every tool for editing every document type, and although you can find a lot of tools out there, not all of them will fit your particular requirements. DocHub makes it much simpler than ever to make and modify, and manage papers - and not just in PDF format.
Every time you need to swiftly revise evidence in WRF, DocHub has got you covered. You can easily alter document components such as text and pictures, and structure. Personalize, arrange, and encrypt documents, create eSignature workflows, make fillable documents for smooth information gathering, etc. Our templates option allows you to create templates based on papers with which you frequently work.
Moreover, you can stay connected to your go-to productivity features and CRM platforms while managing your documents.
One of the most remarkable things about using DocHub is the option to deal with document tasks of any difficulty, regardless of whether you need a fast edit or more diligent editing. It comes with an all-in-one document editor, website document builder, and workflow-centered features. Moreover, you can be sure that your papers will be legally binding and comply with all protection frameworks.
Cut some time off your projects by leveraging DocHub's features that make handling documents straightforward.
welcome to Future consideration in m to focus on real world data and real world evidence and its application in M divided into four sections the first one would be introduction to re data real world evidence in section two weamp;#39;ll look at real world data landscape section three weamp;#39;ll look at application of real word data real word evidence in M section four weamp;#39;ll look at framework for evaluating reward um data reward evidence in regulatory decisions letamp;#39;s look at the definition for real world data and real world evidence real world data is essentially data relating to Patient health status also itamp;#39;s the data that is collected routinely in healthcare settings from a variety of different sources these sources can include doctoramp;#39;s notes information submitted to an insurer disease Registries mobile tech such as variables and sensors and also social media realwood evidence on the other hand is the clinical evidence regarding the usage potential