
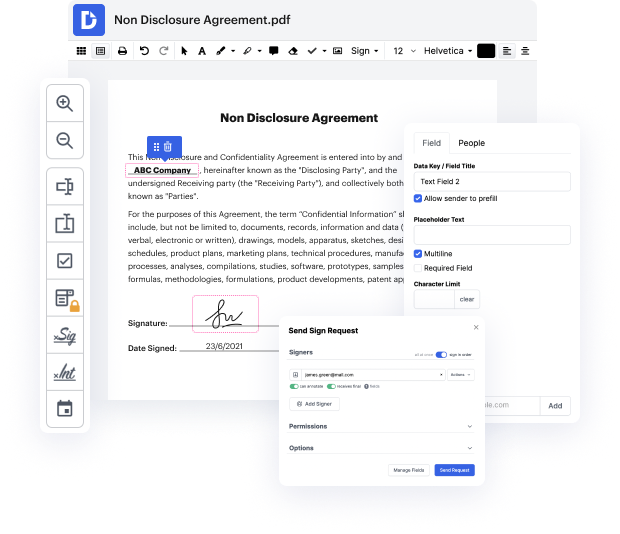
DocHub makes it quick and simple to revise data in FDX. No need to instal any extra application – simply upload your FDX to your account, use the easy drag-and-drop user interface, and quickly make edits. You can even work on your desktop or mobile device to adjust your document online from any place. That's not all; DocHub is more than just an editor. It's an all-in-one document management solution with form creating, eSignature features, and the option to let others fill in and eSign documents.
Every file you edit you can find in your Documents folder. Create folders and organize records for easier search and access. In addition, DocHub ensures the security of all its users' information by complying with stringent security standards.
what can we expect in terms of timing for the next milestone in this review process yeah um as far as we are involved and as far as we know um the um adequacy decision um indeed have been published on 13th of December as youamp;#39;ve mentioned and has immediately been sent to European data protection board um so um the the common Committee of um all Europeans data protection supervisory authorities and um parallel to that and to the European Parliament and to um a kind of Committee of member states so these three groups will all provide comments on that drug adequacy decision and those comments are not binding so in the end we can be pretty sure I guess that there will be a final adequacy decision and because the political will is there and at least the political leaders all do desperately want um an adequacy decision um but nevertheless um those statements can be um important um maybe um some changes are made um ing to the details um and those statements can and give us an Outlook o