
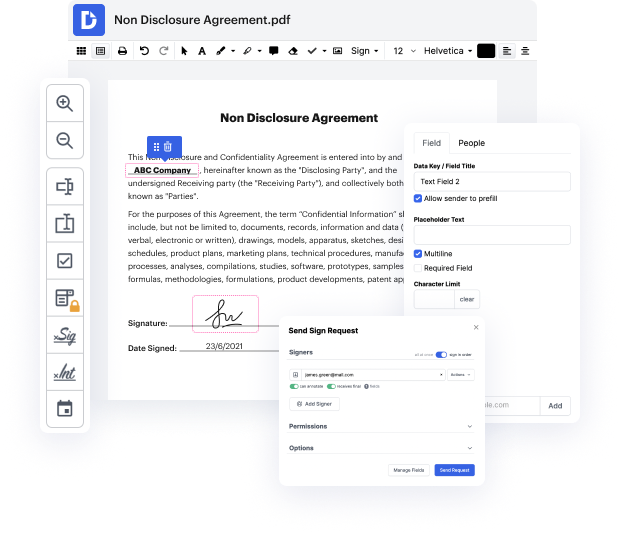
Time is an important resource that every company treasures and attempts to change in a gain. When selecting document management software program, take note of a clutterless and user-friendly interface that empowers customers. DocHub provides cutting-edge instruments to optimize your file management and transforms your PDF file editing into a matter of a single click. Replace Signature via QR Code from the Clinical Trial Agreement with DocHub in order to save a ton of efforts and boost your productivity.
Make PDF file editing an simple and intuitive operation that will save you plenty of valuable time. Effortlessly modify your files and give them for signing without the need of switching to third-party software. Focus on pertinent duties and improve your file management with DocHub starting today.
[Music] this is a demonstration of how to use the practical guidance life sciences practice area to prepare agreements related to the many facets of clinical research for drugs and medical devices one of the fastest and easiest ways to find tools for drafting clinical research agreements is through the clinical trials task on the practical guidance life sciences practice area page here you will find content focused on clinical trials you can get information about fundamentals including contract research organizations irbs and other matters for example this practice note provides drafting tips for contract research organization agreements it also includes a link to cro and research services agreements precedents that will help you to benchmark your agreements with those used by public companies through transaction search by intelligize whether you are tasked with drafting a clinical research support agreement a master clinical trial agreement a material transfer agreement or a sponsored