
Working with documents implies making minor modifications to them daily. Occasionally, the task runs almost automatically, especially if it is part of your everyday routine. However, in other instances, dealing with an uncommon document like a Clinical Trial Agreement Template can take valuable working time just to carry out the research. To ensure that every operation with your documents is trouble-free and swift, you need to find an optimal editing tool for this kind of tasks.
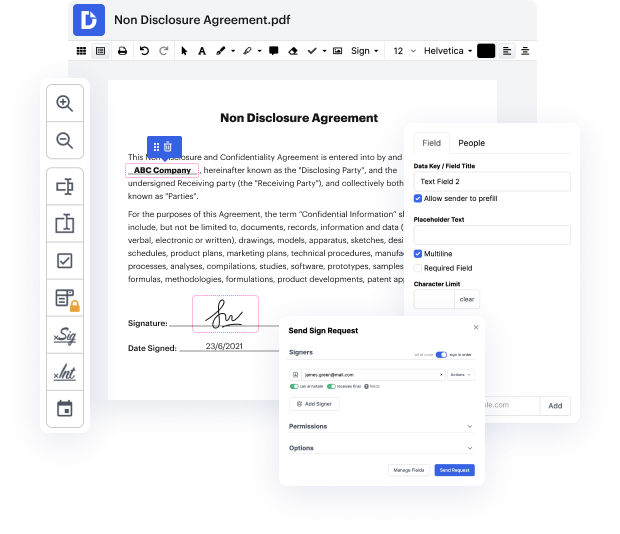
With DocHub, you can see how it works without spending time to figure it all out. Your tools are organized before your eyes and are easily accessible. This online tool does not require any specific background - education or expertise - from its users. It is ready for work even if you are not familiar with software traditionally used to produce Clinical Trial Agreement Template. Quickly make, edit, and share documents, whether you work with them every day or are opening a brand new document type the very first time. It takes moments to find a way to work with Clinical Trial Agreement Template.
With DocHub, there is no need to research different document kinds to figure out how to edit them. Have all the go-to tools for modifying documents close at hand to improve your document management.
In this episode of ClemViz, the focus is on the challenges of CTA negotiations in the clinical trials industry, affecting both sponsors and sites. The ultimate impact is on patients and the timely availability of medications. The speaker believes that CTA negotiation delays are multi-faceted and involve various factors or "villains." A book on this topic is set to be released soon, with upcoming installments discussing the different components contributing to CTA delays.