
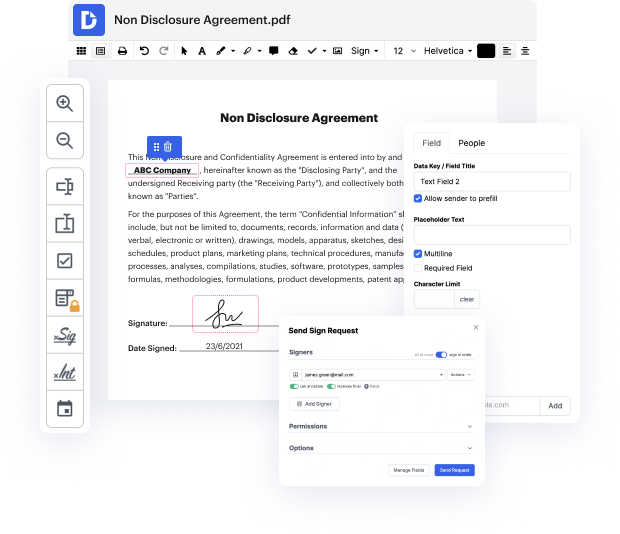
Time is a crucial resource that every enterprise treasures and attempts to turn in a reward. When choosing document management application, pay attention to a clutterless and user-friendly interface that empowers users. DocHub offers cutting-edge tools to improve your file managing and transforms your PDF file editing into a matter of a single click. Insert Field Settings from the Clinical Trial Agreement with DocHub to save a lot of efforts and improve your efficiency.
Make PDF file editing an simple and intuitive process that will save you a lot of precious time. Effortlessly adjust your documents and send them for signing without having adopting third-party alternatives. Give attention to pertinent duties and increase your file managing with DocHub starting today.
um Im excited today to kick off the fall clinical trials toolkit series um our first one is the office of sponsored programs clinical trial agreement review and negotiation um today we have Rachel humberson she is the interim assistant director um from research office and office of sponsored programs and we also have um Julie even though I practiced it Julie Im going to mess it up Anna okay is that right yeah okay she is a negotiator with research with the research office and office of sponsored programs um before I read their um their bios and a fun fact about them again we are offering continuing education to WVU employees so if um you would like CES please indicate that and please put your name um an email address in the chat so for attendance purposes um Im trying to think if theres anything else oh if you have a question during this um feel free to put it in the chat well be watching it and um I will stop Rachel and Julie and let them know what your question is and then also