
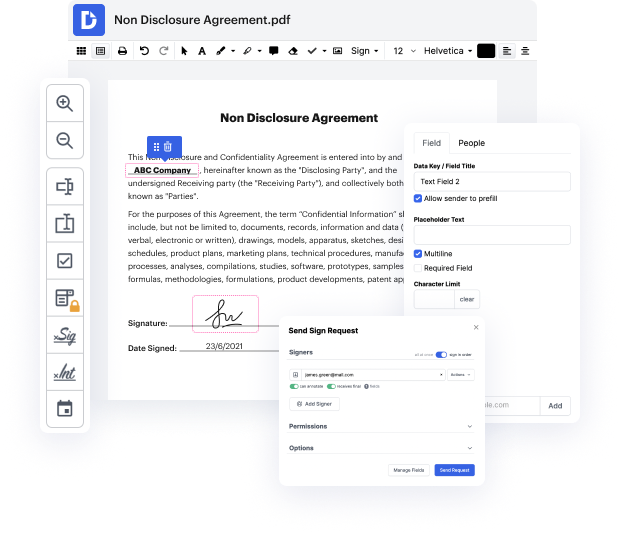
Disadvantages exist in every tool for editing every file type, and despite the fact that you can use a wide variety of tools out there, not all of them will suit your particular requirements. DocHub makes it easier than ever to make and alter, and manage paperwork - and not just in PDF format.
Every time you need to easily inlay certification in csv, DocHub has got you covered. You can quickly modify form elements including text and images, and layout. Personalize, organize, and encrypt files, develop eSignature workflows, make fillable forms for smooth data collection, and more. Our templates feature enables you to create templates based on paperwork with which you frequently work.
In addition, you can stay connected to your go-to productivity capabilities and CRM platforms while managing your files.
One of the most remarkable things about leveraging DocHub is the ability to manage form activities of any complexity, regardless of whether you require a fast tweak or more diligent editing. It includes an all-in-one form editor, website document builder, and workflow-centered capabilities. In addition, you can be sure that your paperwork will be legally binding and abide by all security protocols.
Cut some time off your projects with the help of DocHub's features that make managing files easy.
storing electronic documents seems simple but if your laboratory operates under regulatory requirements such as CLIA ISO GXP or similar regulations or guidance then you know that keeping good digital records can get complicated fast thatamp;#39;s why we offer a comprehensive computer system validation CSV or risk-based computer software assessment CSA of your Thermo Fisher Scientific instrument software this will help your laboratoryamp;#39;s data Integrity policy or procedures follow relevant industry guidance such as 21 CFR part 11 and X11 Gap or similar local regulations our Consultation Services support your data Integrity procedures and help ensure that your data is preserved as complete accurate secure and reproducible electronic data records comprehensive flexible and audit ready our documentation packages reduce guesswork and uncertainty and replace them with the confidence of knowing that your electronic records comply with relevant regulations and standards plus our