
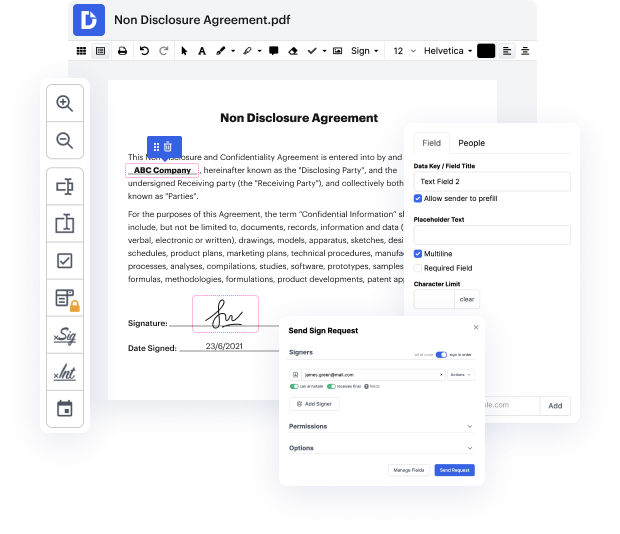
Time is a vital resource that each organization treasures and attempts to turn in a gain. When choosing document management software program, focus on a clutterless and user-friendly interface that empowers consumers. DocHub gives cutting-edge features to enhance your file managing and transforms your PDF file editing into a matter of a single click. Hide Digital Signature into the Clinical Trial Agreement with DocHub to save a ton of time as well as increase your productiveness.
Make PDF file editing an simple and easy intuitive operation that helps save you a lot of precious time. Effortlessly modify your files and send them for signing without having switching to third-party solutions. Focus on pertinent tasks and enhance your file managing with DocHub right now.
today we are very fortunate having uh michael slocum join us he is the senior member of the law firm sloka momboti specializing in contract law legal issues related to clinical applied and basic research corporate issues intellectual property and executive estate planning he has more than 40 years of experience and has represented hospital systems hundreds of businesses both in the us and in foreign countries universities nonprofit organizations and even agencies of the federal government mr slocum negotiates clinical trials and other research contract terms with drug companies and other entities on a daily basis mr slocum began began private practice with his own law firm in 1980 he is an honor graduate of the depauw university and received his jd with honors from george washington university national law center he is a member of the virginia state law bar he is a distinguished faculty member of the society of research administrators and an adjunct professor and graduate school of nur