
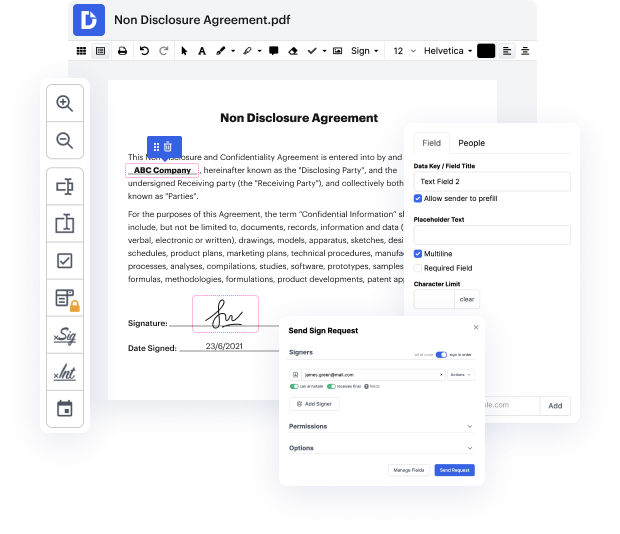
DocHub offers everything you need to quickly change, create and handle and safely store your Clinical Trial Agreement Template and any other paperwork online within a single tool. With DocHub, you can avoid form management's time-consuming and effort-intensive operations. By getting rid of the need for printing and scanning, our environmentally-friendly tool saves you time and minimizes your paper usage.
Once you’ve registered a DocHub account, you can start editing and sharing your Clinical Trial Agreement Template in no time with no prior experience needed. Discover a number of advanced editing tools to finish word in Clinical Trial Agreement Template. Store your edited Clinical Trial Agreement Template to your account in the cloud, or send it to clients using email, dirrect link, or fax. DocHub enables you to turn your form to popular document types without toggling between applications.
You can now finish word in Clinical Trial Agreement Template in your DocHub account whenever you need and anywhere. Your documents are all saved in one platform, where you’ll be able to change and manage them quickly and easily online. Give it a try now!
In this introductory session for the fall clinical trials toolkit series, the focus is on the Office of Sponsored Programs' clinical trial agreement review and negotiation. Presenting are Rachel Humberson, the interim assistant director, and Julie, a negotiator from the research office. The webinar offers continuing education credits for WVU employees, who are encouraged to provide their names and email addresses in the chat for attendance. Attendees are invited to submit questions during the session, which will be relayed to the speakers. The hosts emphasize participation and engagement throughout the tutorial.