
Document creation is a essential part of effective firm communication and administration. You need an affordable and practical solution regardless of your papers preparation stage. Clinical Trial Agreement Template preparation could be among those procedures that need additional care and focus. Simply explained, you will find greater possibilities than manually producing documents for your small or medium organization. One of the best strategies to make sure good quality and effectiveness of your contracts and agreements is to adopt a multifunctional solution like DocHub.
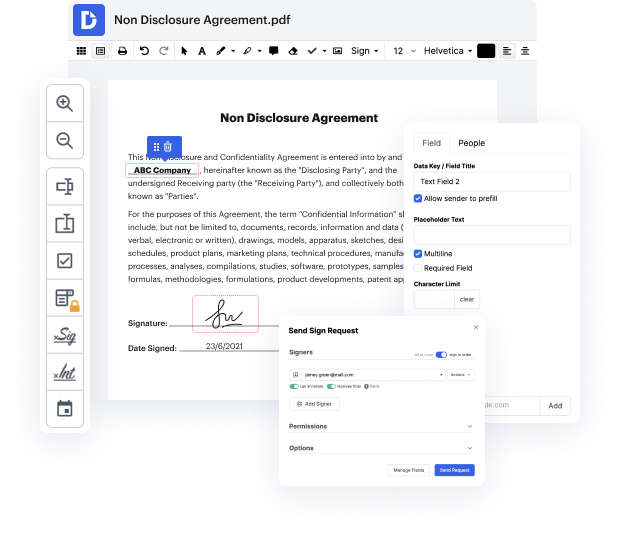
Editing flexibility is considered the most considerable benefit of DocHub. Make use of strong multi-use tools to add and remove, or change any element of Clinical Trial Agreement Template. Leave comments, highlight important info, finish flag in Clinical Trial Agreement Template, and change document management into an easy and intuitive procedure. Gain access to your documents at any moment and apply new adjustments whenever you need to, which may substantially lower your time developing exactly the same document completely from scratch.
Generate reusable Templates to make simpler your everyday routines and steer clear of copy-pasting exactly the same details continuously. Alter, add, and modify them at any moment to ensure you are on the same page with your partners and clients. DocHub can help you avoid errors in frequently-used documents and provides you with the very best quality forms. Make sure that you always keep things professional and remain on brand with the most used documents.
Benefit from loss-free Clinical Trial Agreement Template modifying and safe document sharing and storage with DocHub. Don’t lose any files or find yourself puzzled or wrong-footed when discussing agreements and contracts. DocHub enables professionals everywhere to implement digital transformation as part of their company’s change administration.
[Music] this is a demonstration of how to use the practical guidance life sciences practice area to prepare agreements related to the many facets of clinical research for drugs and medical devices one of the fastest and easiest ways to find tools for drafting clinical research agreements is through the clinical trials task on the practical guidance life sciences practice area page here you will find content focused on clinical trials you can get information about fundamentals including contract research organizations irbs and other matters for example this practice note provides drafting tips for contract research organization agreements it also includes a link to cro and research services agreements precedents that will help you to benchmark your agreements with those used by public companies through transaction search by intelligize whether you are tasked with drafting a clinical research support agreement a master clinical trial agreement a material transfer agreement or a sponsore