
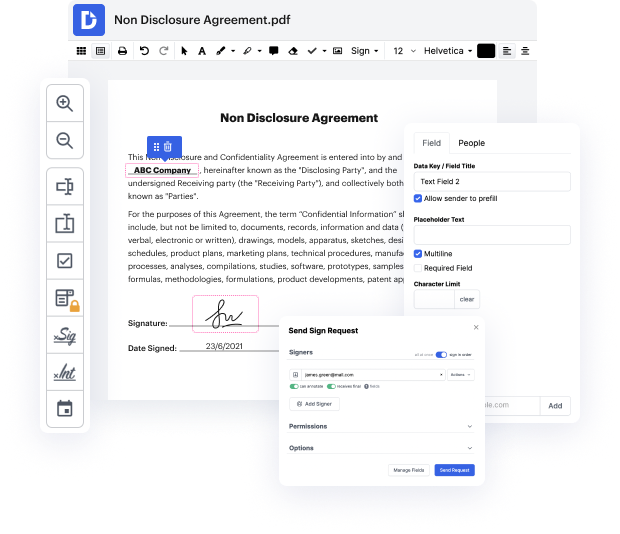
Having comprehensive control of your files at any time is vital to alleviate your day-to-day tasks and boost your productivity. Accomplish any goal with DocHub features for papers management and hassle-free PDF file editing. Gain access, modify and save and integrate your workflows along with other safe cloud storage.
DocHub offers you lossless editing, the opportunity to use any format, and securely eSign papers without looking for a third-party eSignature software. Make the most from the file management solutions in one place. Consider all DocHub capabilities today with your free of charge profile.
In this tutorial, the focus is on using Celebrate Analytics for eDiscovery cases where certain data types are restricted. The presenter demonstrates how to filter out specific data by selecting unwanted categories, such as calls and account information, and applying those filters. This allows users to omit these artifacts from their analysis. Additionally, for cases involving deleted files, users can filter to display all deleted items except for the excluded categories. Overall, the tutorial emphasizes the ability to customize data views in analytics to streamline the analysis process.