
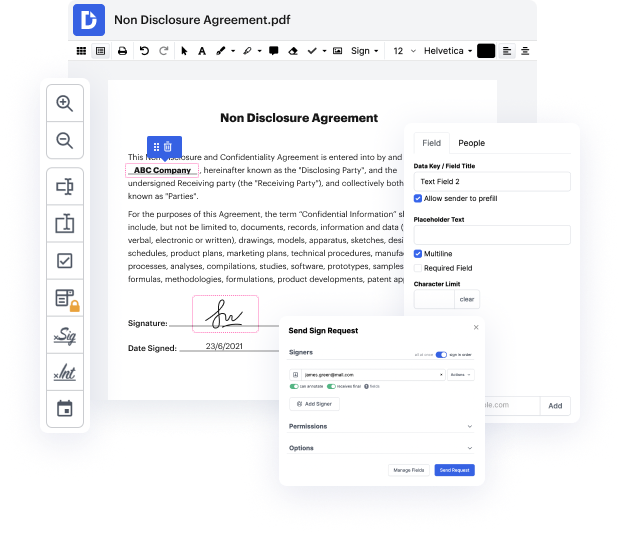
Getting complete control of your files at any moment is important to alleviate your day-to-day tasks and boost your efficiency. Accomplish any objective with DocHub features for papers management and practical PDF editing. Access, change and save and incorporate your workflows with other safe cloud storage.
DocHub offers you lossless editing, the chance to work with any formatting, and safely eSign papers without looking for a third-party eSignature alternative. Make the most of your file managing solutions in one place. Consider all DocHub functions right now with the free of charge profile.
Good afternoon, everyone. I'm David Glaser, here to provide introductions and announcements before turning it over to Bob and Katherine. Our next webinar is scheduled for August 12th, focusing on lessons from recent data breaches, which have resulted in significant, seven-figure losses. Presenters Katie Ilton, Briar Andreessen, and Lad will discuss HIPAA compliance, emphasizing the importance of involving privacy and security personnel. Key topics will include vendor hiring due diligence following breaches, strategies for handling data breaches, tips for navigating OCR audits and settlements, and the development of effective security policies. This insightful session will take place at the same time on August 12th.