


COVID forever transformed how firms view their internal practices and procedures. It influenced companies of all sizes and sectors, posing new challenges for staying connected. The pandemic demonstrated that all firms should incorporate digital instruments into everyday routines. They became essential for far more than hybrid working models.
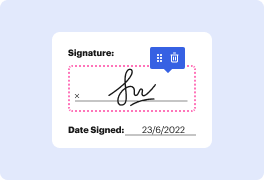
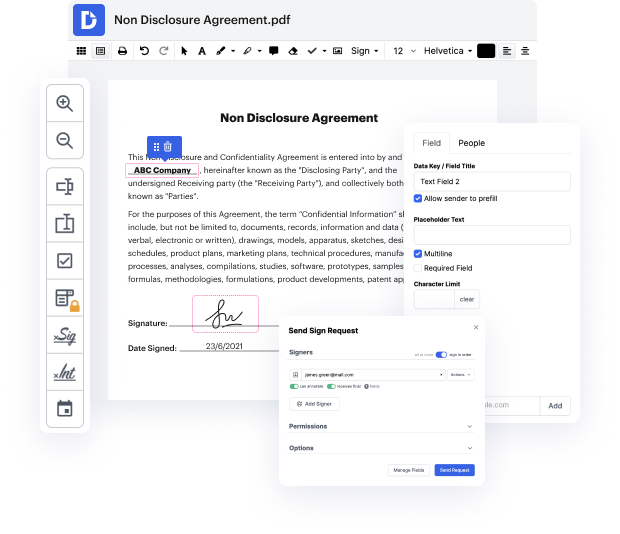
Platforms like DocHub enable you to increase your file administration and approval operations. DocHub is your go-to tool for end-to-end online editing and signatures. It eases your everyday contract and agreement generation and approval tasks. Get access to Docx Software for Pharma | Pharma Document Management Solution superior editing tools that cover all your management demands. Deal with any file type and formatting, create fillable fields, and effectively collect signatures from your teammates and customers. No previous training or experience is necessary.
With Docx Software for Pharma | Pharma Document Management Solution, you are able to maximize the quality of your files, speed up the approval process, and safely store complete files. Get a free DocHub account today and change your plan when you want.
Today's video tutorial focuses on 21 CFR part 11 compliance for pharmaceutical software development and documentation. The video covers regulatory requirements from both European legislative authorities and the FDA, as well as the list of documents needed for software development and validation. The discussion also includes current trends in common problems faced during audits, providing valuable insights for planning software development with your vendor. Subscribe to the YouTube channel for updates and thank you for your support on the Facebook page. Let's delve into these important topics together.
At DocHub, your data security is our priority. We follow HIPAA, SOC2, GDPR, and other standards, so you can work on your documents with confidence.
Learn more



