


COVID forever transformed how organizations view their internal practices and procedures. It impacted organizations of all sizes and industries, posing new challenges for staying connected. The pandemic demonstrated that all organizations should integrate digital tools into everyday routines. They became vital for far more than hybrid working models.
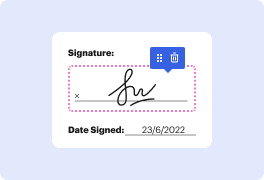
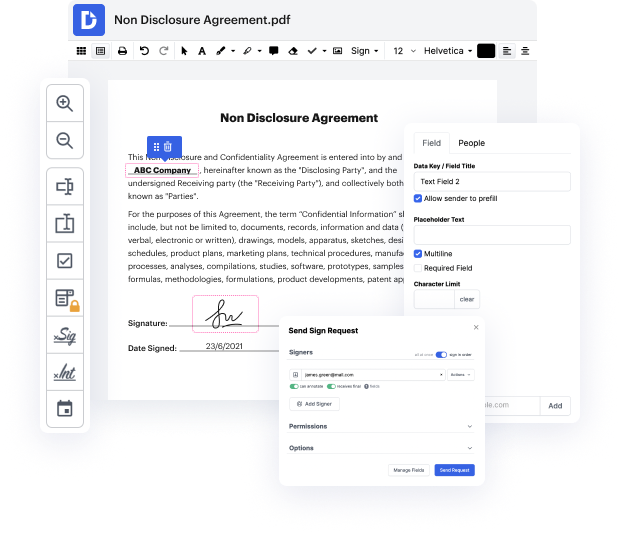
Apps like DocHub enable you to increase your file administration and approval procedures. DocHub is the go-to tool for end-to-end online editing and signatures. It helps in reducing your daily contract and agreement generation and approval tasks. Obtain access to Docx App for Medical Device Manufacturers | Medical Device Manufacturers Document Management Solution superior editing features which cover all your administration requires. Work with any file type and formatting, make fillable fields, and effectively gather signatures from your colleagues and customers. No prior training or experience is necessary.
With Docx App for Medical Device Manufacturers | Medical Device Manufacturers Document Management Solution, it is possible to maximize the quality of your files, boost the approval process, and securely store finished files. Get a cost-free DocHub account today and upgrade your subscription when you want.
In this video tutorial, Peter Sibelius from medicaladvicehq.com provides an overview of the documentation required for a medical device development project. He walks viewers through the process, emphasizing the importance of understanding how everything fits together. This video is part of his online course on design control for medical devices, and he encourages viewers to subscribe for updates. It is essential to note that the information presented is a simplified version of the real process, and individual situations may vary. The goal is to provide a clear perspective on the typical document deliverables needed for medical device product development.
At DocHub, your data security is our priority. We follow HIPAA, SOC2, GDPR, and other standards, so you can work on your documents with confidence.
Learn more



