
COVID forever changed how firms view their internal protocols and procedures. It affected companies of all sizes and industries, posing new obstacles for staying connected. The pandemic demonstrated that all firms need to integrate digital instruments into everyday routines. They became important for far more than hybrid working models.
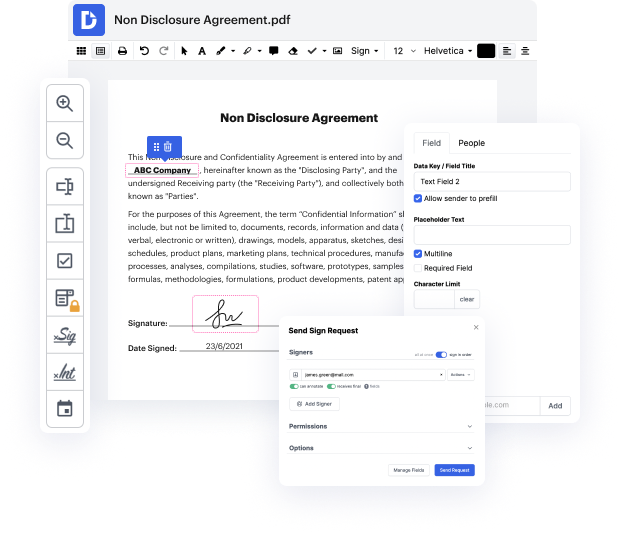
Platforms like DocHub enable you to enhance your document administration and approval processes. DocHub is the go-to instrument for end-to-end online editing and signatures. It helps reduce your daily contract and agreement generation and approval tasks. Obtain access to Doc Tool - Validation Authority superior editing tools that cover all your administration requires. Deal with any document type and formatting, generate fillable fields, and successfully collect signatures from your colleagues and clients. No past training or experience is necessary.
With Doc Tool - Validation Authority, you are able to increase the quality of your documents, accelerate the approval process, and securely store complete documents. Get yourself a free DocHub account right now and change your plan when you want.
Rob Packard from Medical Device Academy introduces the newest procedure, SYS-051, for software tool validation. Written by Mary Vader, this procedure is specifically for software tools utilized in quality systems. It is separate from the existing software validation procedure. The video takes viewers through the procedure, highlighting references to requirements, inputs, outputs, and revision history provided within SYS-051.