
COVID forever altered how firms view their internal protocols and procedures. It affected organizations of all sizes and industries, posing new obstacles for staying connected. The pandemic showed that all firms should incorporate digital tools into daily routines. They became crucial for far more than hybrid working models.
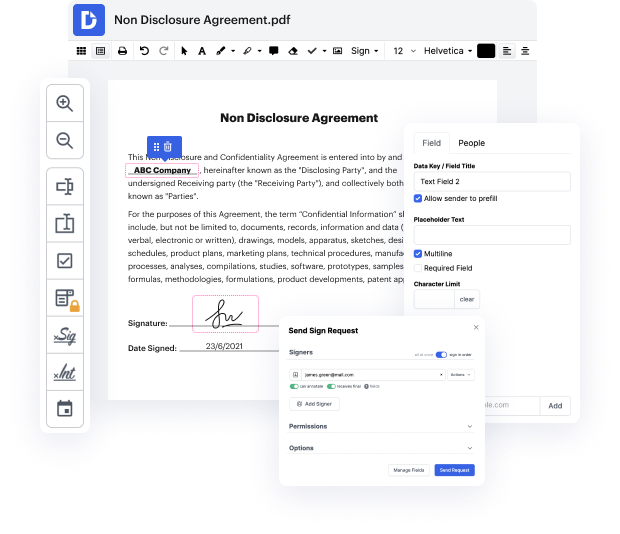
Platforms like DocHub make it easier to improve your document administration and approval procedures. DocHub is the go-to instrument for end-to-end online editing and signatures. It helps reduce your daily contract and agreement generation and approval tasks. Gain access to Doc Tool for Medical Device Manufacturers | Medical Device Manufacturers Document Management Solution sophisticated editing tools which cover all your administration requires. Work with any document type and format, generate fillable fields, and efficiently gather signatures from your colleagues and clients. No previous training or experience is necessary.
With Doc Tool for Medical Device Manufacturers | Medical Device Manufacturers Document Management Solution, it is possible to maximize the quality of your documents, speed up the approval process, and safely store finished documents. Get yourself a cost-free DocHub account right now and upgrade your subscription when ready.
In this video tutorial, Peter Sibelius, the founder of MedicalAdviceHQ.com, provides an overview of the documentation required for a medical device product development project. He walks viewers through how everything fits together, emphasizing the importance of understanding the big picture. The goal is to provide a clear view of the typical document deliverables needed for design control in medical devices. Viewers are invited to check out his online course for a more detailed understanding of the process. Subscribe for notifications on new content.