
COVID forever transformed how businesses look at their internal protocols and procedures. It influenced organizations of all sizes and industries, posing new challenges for staying connected. The pandemic indicated that all businesses need to incorporate digital tools into day-to-day routines. They became vital for far more than hybrid working models.
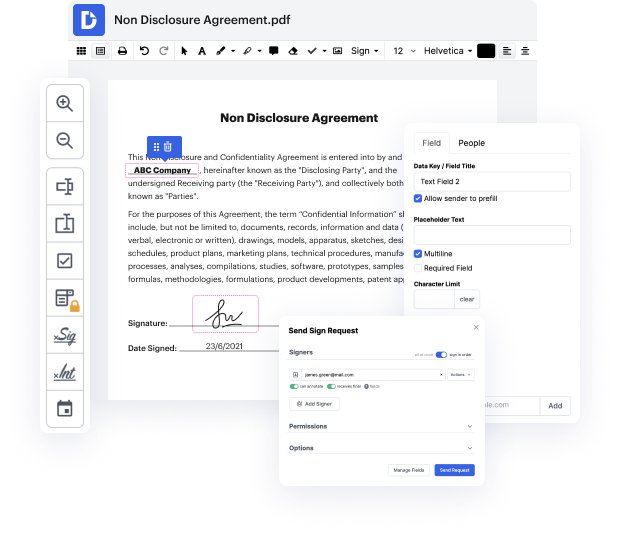
Platforms like DocHub allow you to increase your document management and approval procedures. DocHub is your go-to instrument for end-to-end online editing and signatures. It helps in reducing your day-to-day contract and agreement generation and approval tasks. Gain access to Doc App for Medical Device Manufacturers | Medical Device Manufacturers Document Management Solution innovative editing features that cover all of your managing requires. Deal with any document type and format, make fillable fields, and successfully collect signatures from your colleagues and customers. No prior training or experience is required.
With Doc App for Medical Device Manufacturers | Medical Device Manufacturers Document Management Solution, you can increase the quality of your files, increase the approval process, and safely store complete files. Get yourself a free DocHub account right now and change your plan when ready.
This video tutorial provides a comprehensive overview of the documentation required for a medical device development project. The speaker, Peter Sibelius, walks viewers through how everything fits together and the typical document deliverables needed. The goal is to give a clear understanding of the process, with a focus on design control for medical devices. Viewers are encouraged to subscribe for new content and are reminded that individual situations may vary.