
Not all formats, such as MCW, are developed to be quickly edited. Even though numerous tools can help us edit all form formats, no one has yet created an actual all-size-fits-all tool.
DocHub offers a simple and efficient tool for editing, managing, and storing papers in the most popular formats. You don't have to be a technology-savvy person to darken name in MCW or make other changes. DocHub is robust enough to make the process simple for everyone.
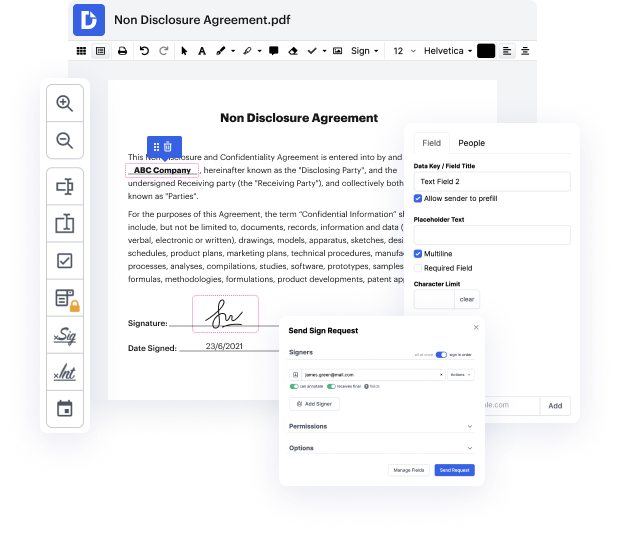
Our feature enables you to change and edit papers, send data back and forth, generate dynamic forms for data gathering, encrypt and protect forms, and set up eSignature workflows. In addition, you can also generate templates from papers you use regularly.
You’ll find plenty of other features inside DocHub, such as integrations that allow you to link your MCW form to different productivity applications.
DocHub is a simple, fairly priced option to handle papers and improve workflows. It provides a wide array of tools, from creation to editing, eSignature providers, and web document creating. The application can export your paperwork in many formats while maintaining greatest protection and following the highest data security standards.
Give DocHub a go and see just how simple your editing transaction can be.
foreign welcome back to my Channel today weamp;#39;re going to be looking at the 2021 paper focusing on the multiple choice section you can find a link to this paper in the description box below letamp;#39;s have a look at question one aluminum carbonate is produced by reacting aluminum chloride and potassium carbonate in a precipitation reaction this produces a solid within a solution a suitable method for separating this would be filtration two is testing your knowledge of Trends in the periodic table weamp;#39;re looking at the difference in size or a covalent radius of sodium and chlorine covalent radius decreases as you move across a period sodium is in group one including itamp;#39;s in group 7. chlorine is smaller than sodium chlorine has more protons than sodium and therefore has an increased nuclear charge this makes the covalent radius smaller covalent radius depends on the number of protons When comparing sodium and chlorine in question 3 weamp;#39;re looking at