
Disadvantages exist in every tool for editing every file type, and even though you can find many solutions out there, not all of them will suit your specific needs. DocHub makes it much simpler than ever to make and alter, and manage documents - and not just in PDF format.
Every time you need to quickly darken formula in WRI, DocHub has got you covered. You can quickly modify form elements including text and images, and structure. Personalize, organize, and encrypt files, develop eSignature workflows, make fillable forms for intuitive information gathering, and more. Our templates feature allows you to create templates based on documents with which you often work.
In addition, you can stay connected to your go-to productivity features and CRM platforms while dealing with your files.
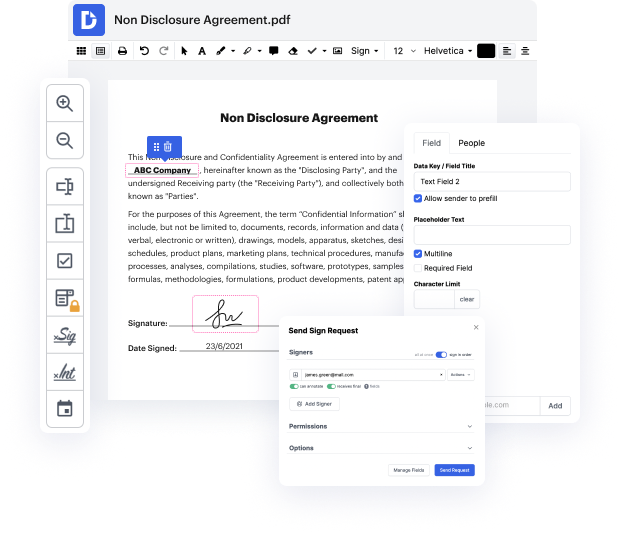
One of the most extraordinary things about utilizing DocHub is the option to handle form activities of any difficulty, regardless of whether you need a quick tweak or more diligent editing. It includes an all-in-one form editor, website document builder, and workflow-centered features. In addition, you can be sure that your documents will be legally binding and comply with all protection frameworks.
Cut some time off your projects by leveraging DocHub's tools that make managing files effortless.
so letamp;#39;s review naming ionic and covalent compounds and make sure that we can tell when to use charges in the crisscross method and when to just use prefixes to let us know what the subscripts should be so weamp;#39;ll start here with iodine hepta fluoride so i have iodine which is a nonmetal and hepta fluoride which is also a nonmetal so iamp;#39;m looking at covalent compounds and i see those prefixes in the name or that one prefix so that tells me that iamp;#39;m looking at a covalent compound so iodine is i and thereamp;#39;s no prefix there which means i only need one and then hepta fluoride so iamp;#39;ll write f for the symbol and hepta is the prefix for seven so iodine heptafloride has the formula if7 so next we have barium oxide barium is a metal and oxide is the name of oxygens negative ion and so i can see that iamp;#39;m looking at an ionic compound so i need to find their charges and then do the crisscross method so barium is in group two so it has a charge o