
Not all formats, such as UOF, are created to be effortlessly edited. Even though a lot of features can help us change all document formats, no one has yet invented an actual all-size-fits-all tool.
DocHub gives a straightforward and efficient tool for editing, handling, and storing paperwork in the most widely used formats. You don't have to be a tech-knowledgeable user to conceal suggestion in UOF or make other changes. DocHub is robust enough to make the process straightforward for everyone.
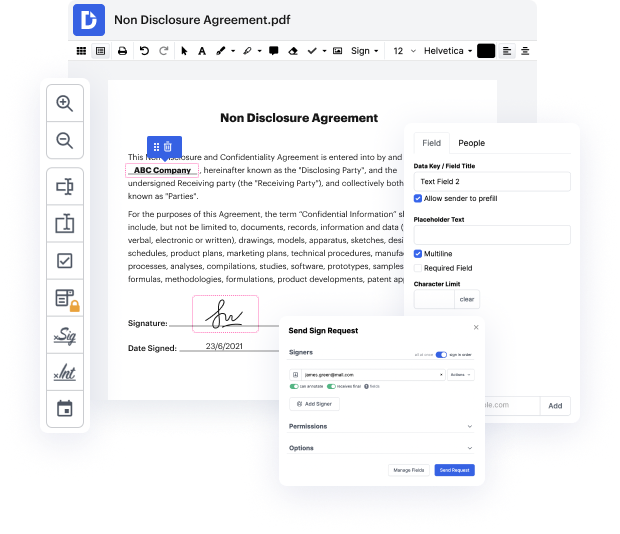
Our feature enables you to alter and tweak paperwork, send data back and forth, generate interactive forms for data collection, encrypt and shield paperwork, and set up eSignature workflows. In addition, you can also create templates from paperwork you utilize regularly.
You’ll find a great deal of additional tools inside DocHub, including integrations that allow you to link your UOF document to a wide array of business applications.
DocHub is an intuitive, fairly priced option to manage paperwork and streamline workflows. It offers a wide array of capabilities, from generation to editing, eSignature services, and web document creating. The software can export your paperwork in multiple formats while maintaining maximum protection and adhering to the greatest data security requirements.
Give DocHub a go and see just how straightforward your editing transaction can be.
hi there dr. Terry shanty fell for UAB School of Medicine concealed allocation is often confused by readers of randomized control trials with blinding in this video Iamp;#39;ll discuss what concealed allocation is and how itamp;#39;s carried out and will also discuss how concealed allocation is critical to maintaining the integrity of the randomization process so letamp;#39;s say you want to do a study to figure out if a blue pill is better than a red pill at preventing death and the best way to do a therapeutic studies to do a randomized control trial so you would randomize patients to a blue pill and red pill and youamp;#39;d have an equal distribution of prognostic factors between those on the blue pill and the red pill but what if you are a clinician or researcher and you had your next patient who potentially could be in this study and youamp;#39;re really concerned about them they were very sick and you wanted to make sure they got the blue pill and letamp;#39;s really consi