
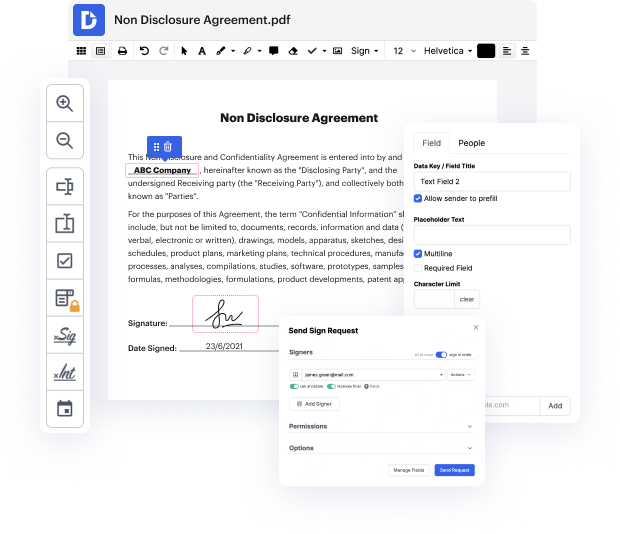
Regardless of how labor-intensive and hard to change your documents are, DocHub delivers a straightforward way to modify them. You can change any part in your XPS without extra resources. Whether you need to fine-tune a single element or the entire form, you can entrust this task to our powerful solution for quick and quality outcomes.
Additionally, it makes sure that the output form is always ready to use so that you can get on with your tasks without any delays. Our all-encompassing set of capabilities also comes with advanced productivity features and a collection of templates, allowing you to take full advantage of your workflows without wasting time on routine activities. On top of that, you can access your documents from any device and integrate DocHub with other apps.
DocHub can handle any of your form management activities. With an abundance of capabilities, you can generate and export papers however you prefer. Everything you export to DocHub’s editor will be saved securely as much time as you need, with strict safety and data security frameworks in place.
Experiment with DocHub today and make managing your paperwork simpler!
in this video Iamp;#39;m going to explain why we use a different labeling system between OJ Peaks and photoelectron Peaks the fundamental reason why we have a different labeling system is that we need to describe the changes in state of an atom or an ion that leads to the emission of either a photoelectron or the emission of an OJ electron the change of state which I refer is the energy that is stored within electrons that are associated with a nucleus that form the atom that is part of the solid state that each electron configuration has its energy and if we remove an electron from the atom then there is a new system with a new energy and the transition from one to the other is what is creating the characteristic energies that we see for these photoelectron Peaks and also the OJ Peaks to understand that the OJ process we must start off by looking at the photo ionization that results in a photoelectron peak if we consider the photo ionization of the copper 2p3 halves then this is achi