
Not all formats, including FDX, are created to be quickly edited. Even though numerous tools will let us change all form formats, no one has yet created an actual all-size-fits-all tool.
DocHub provides a straightforward and streamlined tool for editing, taking care of, and storing papers in the most popular formats. You don't have to be a tech-savvy user to clean up clause in FDX or make other tweaks. DocHub is powerful enough to make the process simple for everyone.
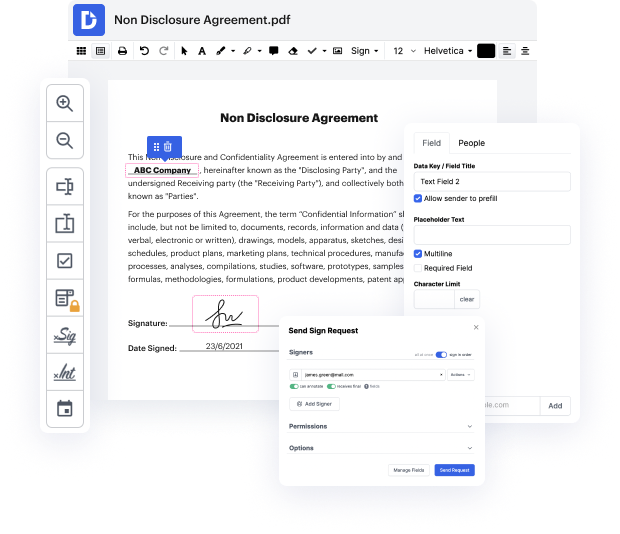
Our feature allows you to change and tweak papers, send data back and forth, generate interactive forms for data collection, encrypt and safeguard documents, and set up eSignature workflows. Moreover, you can also generate templates from papers you use frequently.
You’ll find a great deal of additional tools inside DocHub, including integrations that allow you to link your FDX form to different productivity programs.
DocHub is an intuitive, fairly priced option to handle papers and simplify workflows. It provides a wide selection of features, from creation to editing, eSignature solutions, and web form building. The application can export your documents in many formats while maintaining highest safety and adhering to the greatest data protection requirements.
Give DocHub a go and see just how simple your editing transaction can be.
thank you for the kind introduction my name is jamila harwalka and iamp;#39;m very excited to have the opportunity to speak with you today we are going to discuss why clean rooms and clean room behaviors are important for preventing and sanitary conditions that can adversely impact the quality and safety of drug products for todayamp;#39;s learning objectives we are going to identify the section of the federal food drug and cosmetic act addressing drug products prepared under insanitary conditions we will list different types of filth understand potential sources of filth understand how clean rooms and clean room behaviors prevent or limit the introduction of filth and finally we will discuss multiple examples of 483 observations pertaining to unsanitary conditions so what does the law say section 501a2 of the federal food drug and cosmetic act states that a drug is deemed to be adulterated if it has been prepared packed or held under in sanitary conditions whereby it may have been c