

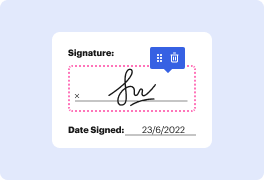

People who work daily with different documents know very well how much efficiency depends on how convenient it is to use editing tools. When you NDA papers have to be saved in a different format or incorporate complex elements, it may be challenging to deal with them utilizing classical text editors. A simple error in formatting may ruin the time you dedicated to change table in NDA, and such a simple job should not feel challenging.
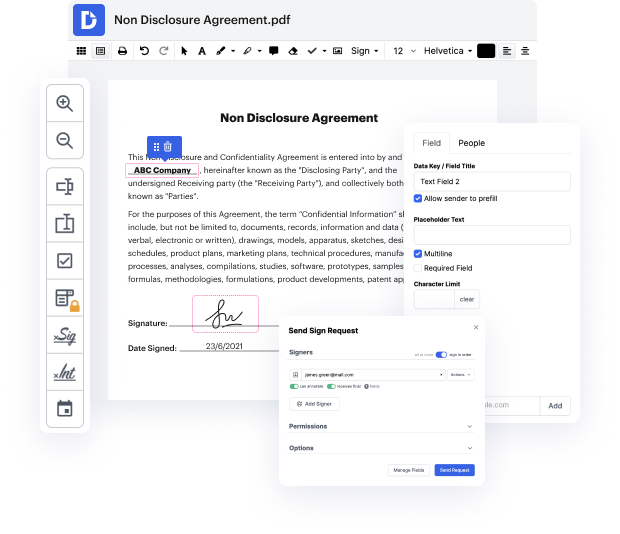
When you find a multitool like DocHub, this kind of concerns will in no way appear in your projects. This powerful web-based editing solution can help you quickly handle documents saved in NDA. You can easily create, modify, share and convert your documents anywhere you are. All you need to use our interface is a stable internet access and a DocHub profile. You can register within minutes. Here is how straightforward the process can be.
Using a well-developed editing solution, you will spend minimal time finding out how it works. Start being productive the moment you open our editor with a DocHub profile. We will make sure your go-to editing tools are always available whenever you need them.
when you break it down and look at it something as simple as a changing table how it could change the way your family does things it's a big deal Dylan is a very free-spirited fun-loving guy okay can you put your feet up he'll be a senior this year we've been in a lot of places that they don't have a place that you can safely and comfortably lay your child down who needs an extra hand you know there's been a lot of let's sit in the truck because we can't go in right now or wait here I've got to change them so just having that peace of mind of him being on a secure table that will hold him and keep him steady while we can take care of him is really awesome it just gives families access to more areas in the community the Rowan Glenn Center we just actually opened in January of 2022 at Brentwood Baptist Church this is our changing table we serve close to 70 families in the community so it was really important for us to have these changing tables in the bathroom so that we can serve those...
At DocHub, your data security is our priority. We follow HIPAA, SOC2, GDPR, and other standards, so you can work on your documents with confidence.
Learn more



