
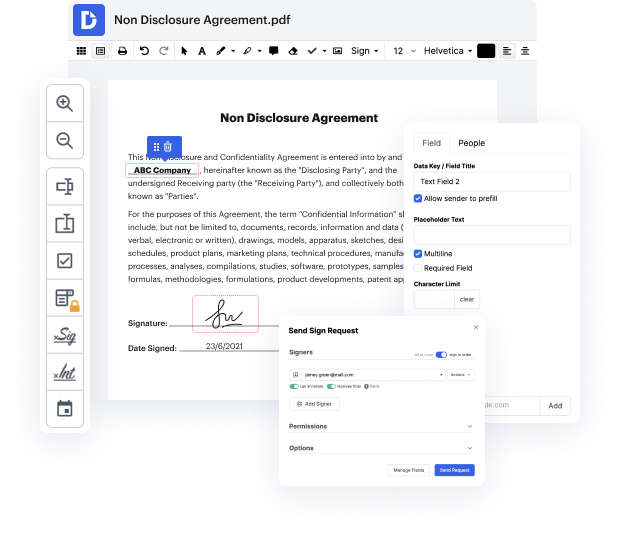
Are you searching for a simple way to change clause in Clinical Trial Agreement Template? DocHub provides the best platform for streamlining form editing, certifying and distribution and form completion. Using this all-in-one online platform, you don't need to download and install third-party software or use multi-level document conversions. Simply upload your form to DocHub and start editing it in no time.
DocHub's drag and drop user interface enables you to swiftly and effortlessly make changes, from simple edits like adding text, graphics, or graphics to rewriting whole form pieces. You can also endorse, annotate, and redact paperwork in just a few steps. The editor also enables you to store your Clinical Trial Agreement Template for later use or transform it into an editable template.
DocHub provides beyond you’d expect from a PDF editing system. It’s an all-encompassing platform for digital form management. You can use it for all your paperwork and keep them safe and easily accessible within the cloud.
hey everyone its dance fair from the clinical trials guru back with yet another video every day I try to do more than one a day but today Im doing this one its a pretty special video somewhat important as I tell you guys all the time you especially you research clinics but this doesnt just apply for to research clinics it applies to everybody in this industry whether you work for a CRO whether youre a p.i wanting to network with other p is whether your site director of a research clinic even sponsors its so important in this industry to network with your colleagues even if its from competing companies even the Big Pharma is doing this now youre seeing a lot of collaboration going on between Big Pharma so theres no reason as research sites should not be able to do this so Im part of of several groups on LinkedIn there are some Google Plus groups theres my special cyber dusters for those of you who dont know cyber dust is an app you can add me dance vera im planning to start