
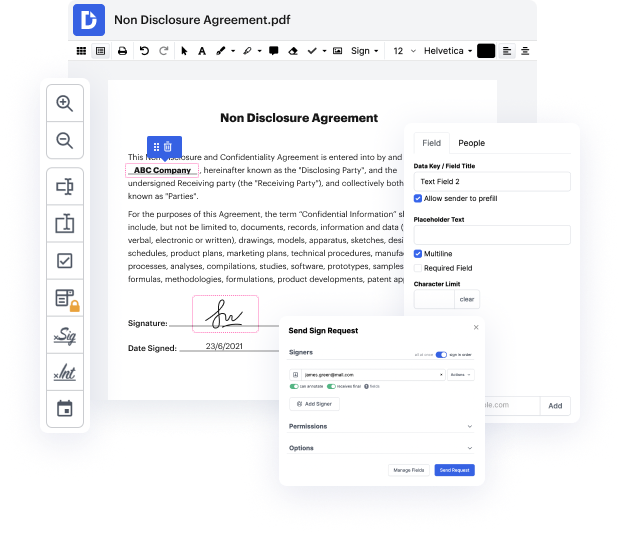
DocHub is an all-in-one PDF editor that lets you blot out chart in MCW, and much more. You can highlight, blackout, or remove paperwork fragments, insert text and pictures where you want them, and collect information and signatures. And because it runs on any web browser, you won’t need to update your software to access its powerful capabilities, saving you money. With DocHub, a web browser is all you need to manage your MCW.
Log in to our website and adhere to these steps:
It couldn't be simpler! Streamline your document management today with DocHub!
which of these elements has the highest melting point while these are all period 3 elements and the pattern as we go across period three looks like this with the three metals on the left hand side then the giant covalent silicon and then the three simple molecular substances and last of all monoatomic argon and so you can see from the diagram that silicon has the highest melting points and the reason is that when weamp;#39;re melting silicon weamp;#39;re breaking a vast number of very strong covalent bonds between these silicon atoms whereas the other three are either simple molecular for sulfur and chlorine or monoatomic for Argon and between those molecules or atoms we only have weak Van Der waals forces which are much easier to overcome than itamp;#39;s the energy required to break the covalent bonds in Silicon so silicon is the correct answer