

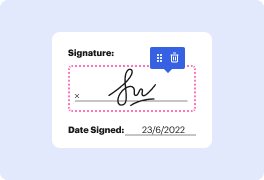

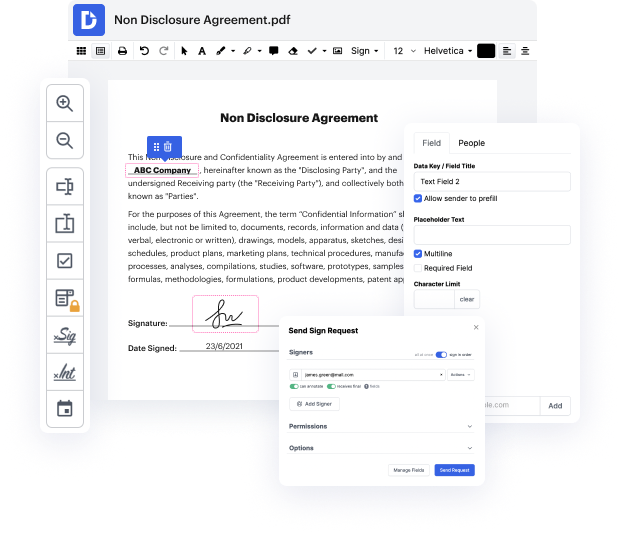
Are you searching for an easy way to black out emblem in Clinical Trial Agreement Template? DocHub offers the best solution for streamlining form editing, certifying and distribution and form endorsement. Using this all-in-one online platform, you don't need to download and set up third-party software or use multi-level file conversions. Simply import your form to DocHub and start editing it with swift ease.
DocHub's drag and drop user interface allows you to easily and effortlessly make modifications, from simple edits like adding text, photos, or visuals to rewriting entire form components. You can also endorse, annotate, and redact paperwork in a few steps. The solution also allows you to store your Clinical Trial Agreement Template for later use or turn it into an editable template.
DocHub offers beyond you’d expect from a PDF editing system. It’s an all-encompassing platform for digital form management. You can utilize it for all your paperwork and keep them secure and easily accessible within the cloud.
The video tutorial marks the start of a fall clinical trials toolkit series, focusing on clinical trial agreement review and negotiation. The session features Rachel Humberson, interim assistant director from the research office, and Julie Anna, a negotiator with the same office. Attendees are encouraged to request continuing education credits by providing their name and email in the chat. Questions can also be submitted in the chat during the presentation, and the host will relay them to Rachel and Julie. The session aims to facilitate understanding of clinical trial agreements and promote engagement among participants.
At DocHub, your data security is our priority. We follow HIPAA, SOC2, GDPR, and other standards, so you can work on your documents with confidence.
Learn more



