
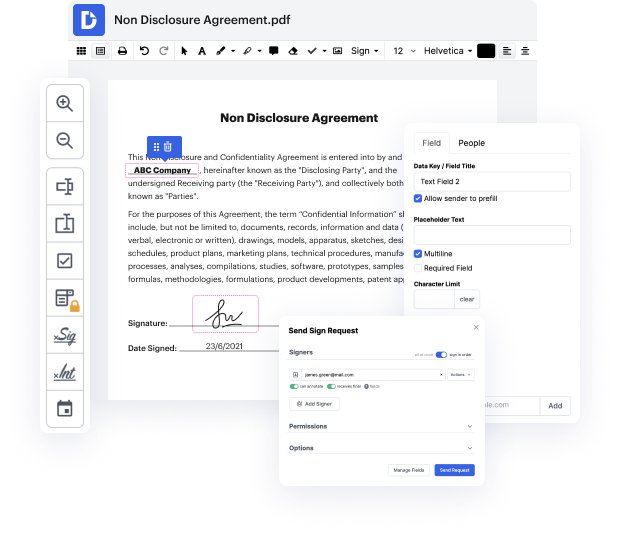
Do you want to avoid the difficulties of editing Quality Incident Record online? You don’t have to worry about installing untrustworthy services or compromising your documents ever again. With DocHub, you can add symbol in Quality Incident Record without spending hours on it. And that’s not all; our user-friendly platform also provides you with highly effective data collection tools for gathering signatures, information, and payments through fillable forms. You can build teams using our collaboration capabilities and effectively work together with multiple people on documents. On top of that, DocHub keeps your data safe and in compliance with industry-leading protection requirements.
DocHub enables you to use its tools regardless of your device. You can use it from your laptop, mobile device, or tablet and modify Quality Incident Record quickly. Begin working smarter right now with DocHub!
Chris Anderberg, Quality and Compliance Manager at SCCR, has over 30 years of research experience in academic and industry settings. Her expertise includes clinical trial management, GCP compliance audits, regulatory inspections, clinical event education, quality management of clinical trials, and policy development. In the tutorial, Chris expresses excitement about discussing quality incident reporting, specifically focusing on KAPPAs, and aims to cover key concepts in the session. She acknowledges a few technical difficulties at the start and thanks Susan for the introduction.