Manage your order documents and explore More 222 Order Forms. Keep sensitive data safe with DocHub's encryption and access controls.












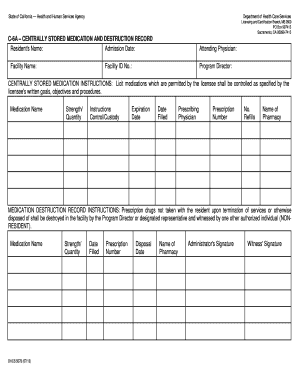




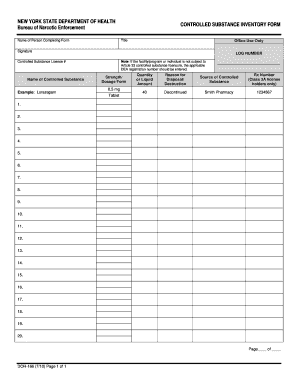


















Document management can overpower you when you can’t find all of the documents you require. Luckily, with DocHub's extensive form collection, you can get everything you need and promptly deal with it without the need of changing among applications. Get our More 222 Order Forms and start working with them.
Using our More 222 Order Forms using these easy steps:
Try out DocHub and browse our More 222 Order Forms category without trouble. Get a free profile today!