Enhance efficiency with our modifiable Dea Order Forms templates. Edit and customize forms to fit your specific business needs in just a few clicks.




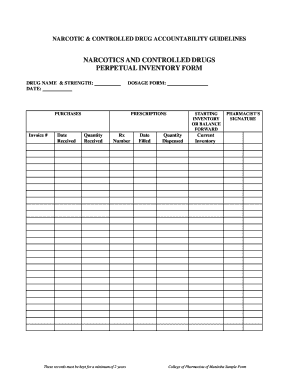















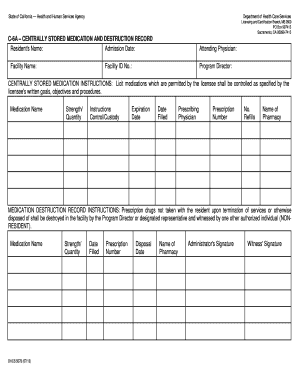






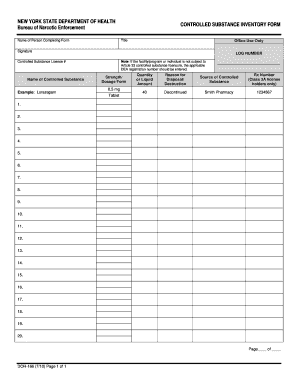


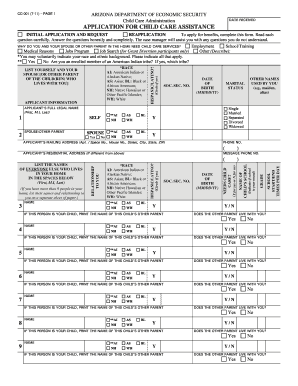





Improve your file operations with our Dea Order Forms online library with ready-made templates that meet your needs. Get the document, alter it, fill it, and share it with your contributors without breaking a sweat. Start working more effectively with your documents.
How to use our Dea Order Forms:
Explore all of the possibilities for your online file administration using our Dea Order Forms. Get a free free DocHub profile right now!