
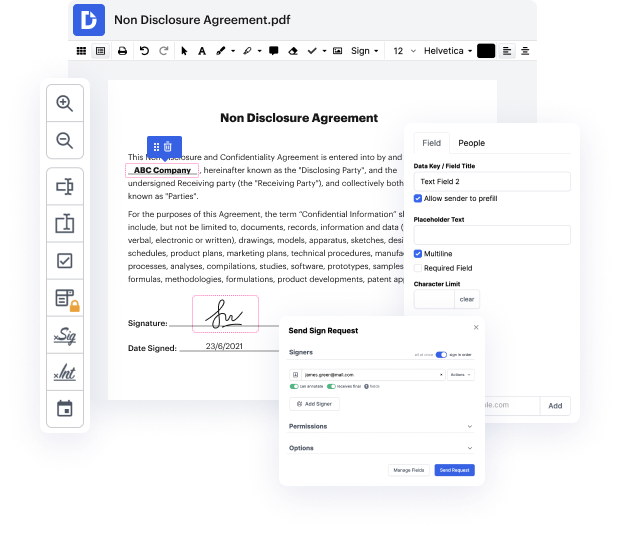
Need to rapidly wipe stain in Clinical Trial Agreement Template? Your search is over - DocHub offers the solution! You can get the work finished fast without downloading and installing any application. Whether you use it on your mobile phone or desktop browser, DocHub enables you to alter Clinical Trial Agreement Template anytime, at any place. Our comprehensive solution comes with basic and advanced editing, annotating, and security features, ideal for individuals and small businesses. We offer lots of tutorials and instructions to make your first experience productive. Here's an example of one!
You don't have to bother about data safety when it comes to Clinical Trial Agreement Template editing. We provide such security options to keep your sensitive data secure and safe as folder encryption, two-factor authentication, and Audit Trail, the latter of which tracks all your actions in your document.
This video tutorial demonstrates how to utilize the Practical Guidance Life Sciences practice area for preparing agreements related to clinical research for drugs and medical devices. It highlights the "clinical trials" task as a quick resource for drafting clinical research agreements. Viewers can access fundamental information on contract research organizations (CROs), institutional review boards (IRBs), and other relevant topics. The tutorial also provides drafting tips for CRO agreements and offers links to precedents for benchmarking agreements against those used by public companies. It covers various agreement types, including clinical research support agreements, master clinical trial agreements, material transfer agreements, and sponsorship agreements.