
Not all formats, including XPS, are designed to be quickly edited. Even though a lot of capabilities can help us edit all form formats, no one has yet invented an actual all-size-fits-all solution.
DocHub offers a simple and efficient solution for editing, taking care of, and storing papers in the most widely used formats. You don't have to be a technology-knowledgeable user to vary recipient in XPS or make other changes. DocHub is robust enough to make the process easy for everyone.
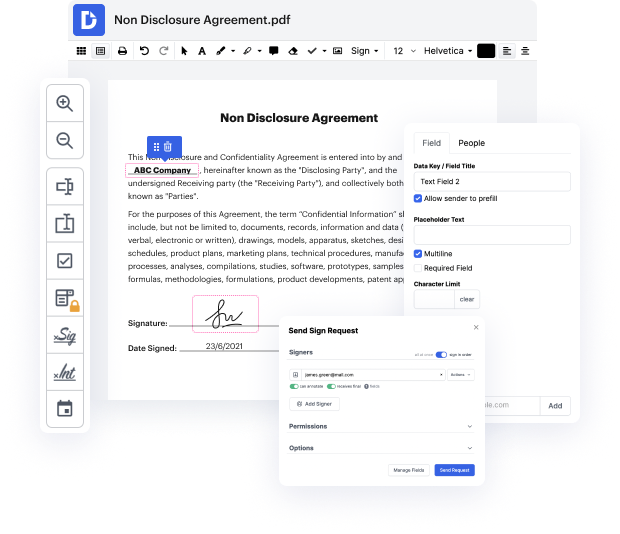
Our feature allows you to alter and tweak papers, send data back and forth, generate interactive forms for data gathering, encrypt and shield forms, and set up eSignature workflows. In addition, you can also create templates from papers you utilize on a regular basis.
You’ll locate a great deal of other features inside DocHub, such as integrations that let you link your XPS form to a wide array of business applications.
DocHub is a straightforward, cost-effective option to handle papers and simplify workflows. It offers a wide array of tools, from creation to editing, eSignature professional services, and web form developing. The program can export your paperwork in many formats while maintaining highest security and adhering to the greatest data security standards.
Give DocHub a go and see just how easy your editing process can be.
hello friends welcome back to the series of photoemission spectra and in this video we will discuss about spin orbital coupling of xps data when we try to analyze the xps data we can see that some of the peaks show sharp single peak like in case of sodium 1s as shown here and in some cases the peak is split into two parts for example as in case of chlorine 2p this peak splitting is observed due to spin orbital coupling as the name suggests it is the coupling or interaction between spin and orbital motion of electron so letamp;#39;s first understand what is spin of electron speed spin is rotation of electron around its own axis and this rotation produces a magnetic field as shown here with the blue line now as electron is also revolving around the nucleus this angular motion also produces a magnetic field we can try to see this orbital motion in an alternate point of view where you can say that the nucleus is moving around the electron itamp;#39;s the same thing but a different perspe