
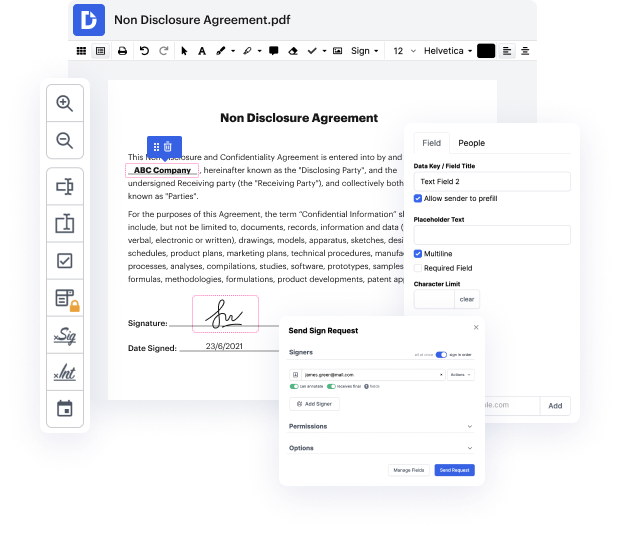
There are so many document editing solutions on the market, but only some are compatible with all file types. Some tools are, on the contrary, versatile yet burdensome to work with. DocHub provides the solution to these challenges with its cloud-based editor. It offers powerful capabilities that enable you to complete your document management tasks effectively. If you need to quickly Vary identification in Odt, DocHub is the ideal choice for you!
Our process is very easy: you upload your Odt file to our editor → it instantly transforms it to an editable format → you apply all necessary changes and professionally update it. You only need a couple of moments to get your work ready.
As soon as all alterations are applied, you can turn your paperwork into a multi-usable template. You just need to go to our editor’s left-side Menu and click on Actions → Convert to Template. You’ll locate your paperwork stored in a separate folder in your Dashboard, saving you time the next time you need the same form. Try DocHub today!
UDi this means unique device identification. Are you an expert on it? Do you know exactly what it is? So I will try to provide you all the information regarding the new feature that comes with the new medical device regulation MDR 2017/745 and also the IVDR 2017/746. So lets dig in. Hey, welcome everyone to the medical device made easy podcast. Im your host Monir El Azzouzi. And my objective is to make it easy for you to place a compliant medical device on the market. And for that we will look at the UDI today, the unique device identification. But before that, just a reminder, if you can subscribe to my podcast just on your preferred platforms like itunes, spotify, or soundcloud, this would be really great for me. So thank you. Thank you very much. Okay, so lets dig in. Now what Ill do today is, I helped you to understand what is the UDI for the medical device market in the European Union. So specifically for the MDR 2017/745, I will try to explain you what it means, how to