
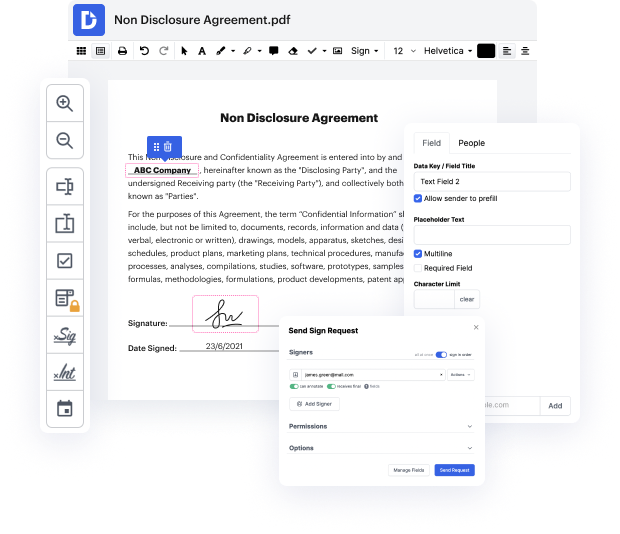
Having comprehensive control of your papers at any time is essential to ease your daily duties and enhance your efficiency. Accomplish any goal with DocHub features for papers management and hassle-free PDF editing. Gain access, adjust and save and integrate your workflows with other protected cloud storage services.
DocHub offers you lossless editing, the chance to work with any formatting, and safely eSign documents without having looking for a third-party eSignature alternative. Obtain the most from the file managing solutions in one place. Check out all DocHub functions right now with the free of charge account.
David Glaser introduces a webinar scheduled for August 12th, focusing on "Lessons from Recent Data Breaches," featuring Katie Ilton and Briar Andreessen. The session will cover significant lessons learned, especially regarding HIPAA compliance and costly data breaches. Key topics will include due diligence when hiring vendors post-breach, tips for managing data breaches, and navigating OCR audits and settlements. Attendees are encouraged to involve privacy and security personnel in the discussion for better insights. Glaser closes with a hint about upcoming news announcements.