
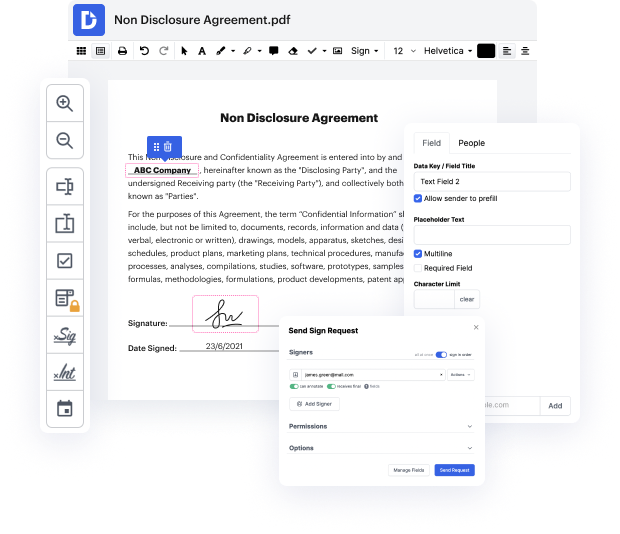
Having comprehensive control of your papers at any moment is essential to ease your everyday duties and increase your efficiency. Achieve any objective with DocHub features for papers management and practical PDF file editing. Gain access, adjust and save and incorporate your workflows with other protected cloud storage services.
DocHub gives you lossless editing, the possibility to work with any formatting, and safely eSign documents without having searching for a third-party eSignature alternative. Obtain the most of the document management solutions in one place. Consider all DocHub functions today with your free account.
This tutorial demonstrates how to utilize the Practical Guidance Life Sciences practice area for preparing agreements related to clinical research for drugs and medical devices. It highlights the Clinical Trials task on the practice area page, which offers tools for drafting clinical research agreements. Users can access information on key topics such as contract research organizations (CROs) and IRBs. The practice note provides drafting tips for CRO agreements and links to precedents for benchmarking against public companies. It covers various agreement types, including clinical research support agreements, master clinical trial agreements, material transfer agreements, and sponsored research agreements.