
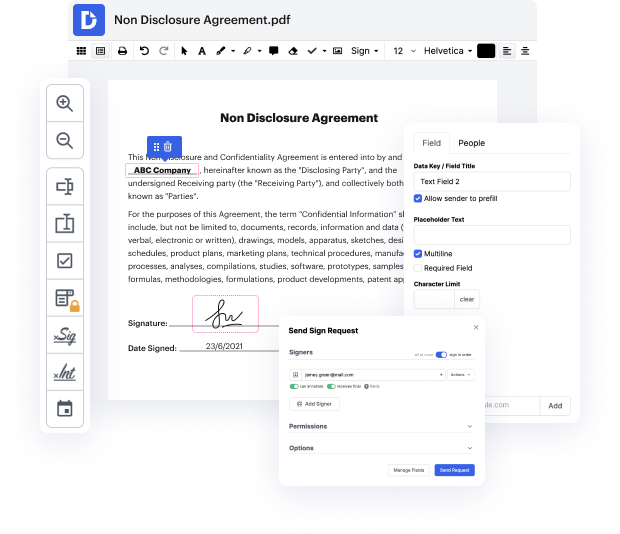
Manual document processing can be quite a cause of your company losing money as well as your staff members losing interest in their duties. The easiest way to speed up all business procedures and boost your statistics would be to take care of everything with cutting-edge software like DocHub. Manage all of your documents and Save Clinical Trial Agreement in Excel within seconds and save more time for relevant duties.
With DocHub, you have unrestricted access to your documents and Templates available to you at any moment. Explore all functionalities today with the free of charge DocHub profile.
Excel Clinical Research conducts clinical trials to investigate drugs for FDA approval. People may enroll in these trials for access to alternative treatments that are not yet available or approved, such as experimental medications for conditions like liver cancer. The trials assess the safety and efficacy of these drugs. Excel also conducts studies related to the flu virus, coronavirus, and skin disorders like eczema. Clinical trials provide opportunities for patients to explore new treatment options that could potentially benefit their medical conditions.