

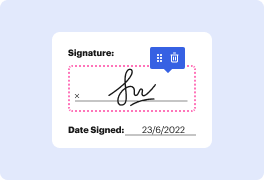

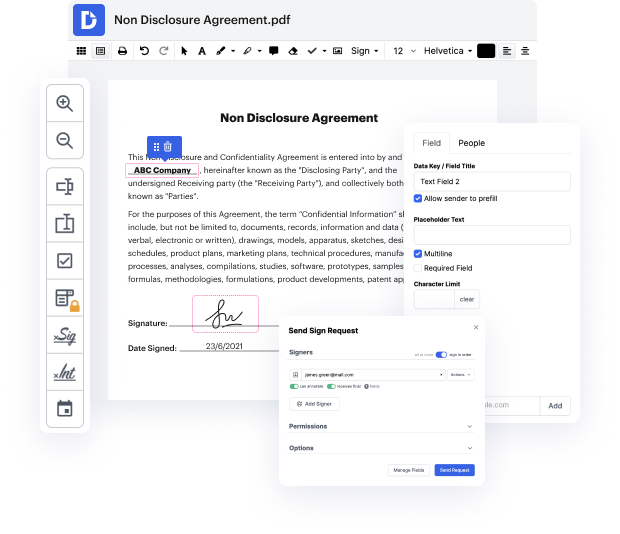
Handling and executing papers can be monotonous, but it doesn’t have to be. Whether you need help everyday or only occasionally, DocHub is here to supply your document-based projects with an extra productivity boost. Edit, comment, complete, sign, and collaborate on your Clinical Trial Agreement Template quickly and effortlessly. You can modify text and pictures, build forms from scratch or pre-made web templates, and add eSignatures. Due to our top-notch safety measures, all your information stays safe and encrypted.
DocHub provides a comprehensive set of tools to streamline your paper workflows. You can use our solution on multiple systems to access your work anywhere and anytime. Improve your editing experience and save hours of handiwork with DocHub. Try it for free right now!
The fall clinical trials toolkit series begins with a focus on the Office of Sponsored Programs Clinical Trial Agreement review and negotiation. Presenters include Rachel Humberson, interim assistant director from the research office, and negotiator Julie (last name unclear). Participants are eligible for continuing education credits, and are encouraged to provide their name and email for attendance tracking. A Q&A session will be facilitated in the chat, allowing attendees to ask questions during the presentation.
At DocHub, your data security is our priority. We follow HIPAA, SOC2, GDPR, and other standards, so you can work on your documents with confidence.
Learn more



